In 2024, the National Health Commission of the People’s Republic of China (NHC) issued four announcements (No. 2 of 2024, No. 3 of 2024, No. 5 of 2024, No. 6 of 2024) regarding “Three New Food”. A total of 54 products, including new food raw materials (novel food), new food additives, and food-related products (new food contact substances) have been approved, 12 of which are new food raw materials (novel food), namely dendrobium protocorm, meso-zeaxanthin, pichia kluyveri, bacillus subtilis DE111, L-alpha-Glycerylphosphorylcholine, leuconostoc pseudomesenteroides, arabinoxylan, bifidobacterium longum subsp. infantis M-63, N-acetylglucosamine, nannochloropsis gaditana oil, procyanidins and camellia petelotii culture.

Furthermore, according to the official announcements issued by the NHC and the China National Center for Food Safety Risk Assessment (CFSA), throughout 2024:
- 30 new food raw materials applications were accepted;
- 13 new food raw materials were issued for public comments;
- 5 products were rejected for approval; and
- 9 substances were included in the termination of review list.
CIRS has summarized the acceptance and approval status of new food raw materials in China in 2024 as follows:
1. List of new food raw materials accepted in 2024 (30 types)
In 2024, NHC accepted the applications for 30 new food raw materials, with 22 domestically produced products and 8 imported products. The detailed technical review status is listed in the table below:
S.N. | Date of acceptance | Acceptance code | Name | Technical review status |
1 | 2024-01-02 | 衛食新申字(2024)第0001號 | Maple acer truncatum | Not issued for public comments yet |
2 | 2024-01-02 | 衛食新進申字(2024)第0001號 | Cockscomb extract | 2024.10.24 Issued for public comments |
3 | 2024-01-02 | 衛食新申字(2024)第0002號 | Gardenia oil | Not issued for public comments yet |
4 | 2024-01-05 | 衛食新申字(2024)第0003號 | Golden camellia cell culture | Not issued for public comments yet |
5 | 2024-03-13 | 衛食新進申字(2024)第0002號 | Saccharomyces cerevisiae CNCM I-3799 | Not issued for public comments yet |
6 | 2024-03-14 | 衛食新申字(2024)第0004號 | Siraitia grosvenorii | Terminated the review |
7 | 2024-03-18 | 衛食新進申字(2024)第0003號 | Lutein ester | Not issued for public comments yet |
8 | 2024-03-21 | 衛食新申字(2024)第0005號 | Safflower seedling | Terminated the review |
9 | 2024-04-29 | 衛食新進申字(2024)第0004號 | Lemon myrtle leaf | 2024.08.28 Issued for public comments |
10 | 2024-05-10 | 衛食新申字(2024)第0006號 | PQQ disodium salt | Not issued for public comments yet |
11 | 2024-05-15 | 衛食新申字(2024)第0007號 | Camelina seed oil | Not issued for public comments yet |
12 | 2024-05-17 | 衛食新申字(2024)第0008號 | Cis-15-Tetracosenoic acid (Synthetic Method) | Rejected for approval |
13 | 2024-05-17 | 衛食新申字(2024)第0009號 | Ergothioneine | Not issued for public comments yet |
14 | 2024-05-20 | 衛食新申字(2024)第0010號 | Bacillus subtilis QK02 | Rejected for approval |
15 | 2024-06-13 | 衛食新申字(2024)第0011號 | Siraitia grosvenorii | Not issued for public comments yet |
16 | 2024-06-20 | 衛食新申字(2024)第0012號 | ‘Xiangyu’ peony flower | Not issued for public comments yet |
17 | 2024-07-02 | 衛食新申字(2024)第0013號 | PQQ disodium salt | Not issued for public comments yet |
18 | 2024-07-09 | 衛食新申字(2024)第0014號 | Enteromorpha polysaccharide | Not issued for public comments yet |
19 | 2024-07-19 | 衛食新申字(2024)第0015號 | Glutathione yeast powder | Not issued for public comments yet |
20 | 2024-07-22 | 衛食新進申字(2024)第0005號 | Cherry blossom extract | 2024.10.24 Issued for public comments |
21 | 2024-07-24 | 衛食新進申字(2024)第0006號 | Bifidobacterium animalis subsp. lactis CBS-118529 | Not issued for public comments yet |
22 | 2024-08-22 | 衛食新進申字(2024)第0007號 | N-Acetylglucosamine (Enzymatic Method) | Not issued for public comments yet |
23 | 2024-09-12 | 衛食新進申字(2024)第0008號 | Bifidobacterium longum subsp. infantis LMG 11588 | Not issued for public comments yet |
24 | 2024-09-13 | 衛食新申字(2024)第0016號 | Nervonic acid oil | Not issued for public comments yet |
25 | 2024-09-14 | 衛食新申字(2024)第0017號 | Chlorella vulgaris (white algae) | Terminated the review |
26 | 2024-09-14 | 衛食新申字(2024)第0018號 | D-Allulose | Not issued for public comments yet |
27 | 2024-09-24 | 衛食新申字(2024)第0019號 | Peony seed oil | Not issued for public comments yet |
28 | 2024-11-8 | 衛食新申字(2024)第0020號 | Bacillus natto VB205 | Recommend not been approved |
29 | 2024-11-21 | 衛食新申字(2024)第0021號 | Dehong gleditsia sinensis | Not issued for public comments yet |
30 | 2024-11-26 | 衛食新申字(2024)第0022號 | Red date leaf | Not issued for public comments yet |
2. List of new food raw materials issued for public comments in 2024 (13 types)
In 2024, 13 new food raw materials have passed the technical review of CFSA and were issued for public comments. (Only the 7 raw materials that are still in the public consultation stage and have not been approved yet are presented below. See Part 3 for detailed information on the other 5 approved raw materials).
Details are as follows:
(1) Rye pollen (Draft announcement)
Name | Rye pollen |
Other information | Rye pollen was approved as a new food raw material by Announcement No. 3 of 2023 issued by the National Health Commission. Based on industry data and after review by the expert evaluation committee, it is now proposed to revise the dietary fiber content requirement in the announcement to ≥ 30%. |
Issued date | Passed the technical review and issued for public comments on April 28, 2024 |
(2) Stevia leaf polyphenols (Draft announcement)
Name | Stevia leaf polyphenols |
Basic information | Source: Stevia rebaudiana Bertoni |
Brief introduction of the production process | Made from the leaves of stevia as raw material, processed through ethanol extraction, filtration, concentration, drying, and other techniques. |
Recommended intake | ≤500 mg/day (count as polyphenols) |
Other information |
|
Acceptance date | Accepted on September 7, 2023. The acceptance code is衛食新申字(2023)第0015號. |
Issued date | Passed the technical review and issued for public comments on June 14, 2024. |
(3) Lemon myrtle leaf (Draft announcement)
Name | Lemon myrtle leaf |
Basic information | Source: Backhousia citriodora F. Muell. |
Brief introduction of the production process | Made from the leaves of lemon myrtle as raw material, processed through harvesting, screening, cleaning, drying, and other techniques. |
Other information | 1. Infants, young children, pregnant women, and breastfeeding women are not recommended to consume this product. Labels and instructions must indicate the unsuitable groups and consumption limits. 2. Food safety indicators shall meet the relevant requirements as outlined in the draft announcement. |
Acceptance date | Accepted on April 29, 2024. The acceptance code is衛食新進申字(2024)第0004號. |
Issued date | Passed the technical review and issued for public comments on August 28, 2024. |
(4) Maqui Berry anthocyanins (Draft announcement)
Name | Maqui Berry anthocyanins |
Basic information | Source: Aristotelia chilensis |
Brief introduction of the production process | Made from the fruit of maqui berry as raw material, processed through water extraction, filtration, purification, concentration, drying, and other techniques. |
Recommended intake | ≤900 mg/day (count as a total anthocyanin content of 35 g/100 g. Any content exceeding this value should be converted according to the actual content.) |
Other information | 1. Scope of use and maximum usage levels: - Milk and dairy products (0.8 g/kg for modified milk and flavored fermented milk, milk powder and modified milk powder calculated based on the liquid mass after reconstitution). - Beverages (0.8 g/kg for liquid beverages, solid beverages calculated based on the liquid mass after reconstitution). - Jelly, cocoa products, chocolate, and chocolate products (including cocoa butter substitute chocolate and its products) (14 g/kg). - Confectionery (40 g/kg). - Frozen desserts (8 g/kg). - Baked goods (4 g/kg). - Alcoholic beverages (4 g/kg). 2. Infants, young children, pregnant women, and breastfeeding women are not recommended to consume this product. Labels and instructions must indicate the unsuitable groups and consumption limits. 3. Food safety indicators shall meet the relevant requirements as outlined in the draft announcement. |
Acceptance date | Accepted on March 29, 2022. The acceptance code is 衛食新進申字(2022)第0001號. |
Issued date | Passed the technical review and issued for public comments on August 28, 2024. |
(5) Wheat polar lipids (Draft announcement)
Name | Wheat polar lipids |
Brief introduction of the production process | Made from wheat flour as the raw material, processed through ethanol extraction, acetone precipitation, separation, drying, and grinding. |
Recommended intake | ≤ 30 mg/day (calculated as digalactosyldiacylglycerol content at 40 g/100 g, any content exceeding this value should be converted according to the actual content.) |
Other information | 1. Scope of use and maximum usage levels: Beverages (0.1 g/kg for liquid beverages, solid beverages calculated based on the liquid mass after reconstitution). 2. Infants, young children, pregnant women, and breastfeeding women are not recommended to consume this product. Labels and instructions must indicate the unsuitable groups and consumption limits. 3. Quality specifications and food safety indicators are detailed in the draft announcement. |
Acceptance date | Accepted on September 22, 2021. The acceptance code is 衛食新進申字(2021)第0007號. |
Issued date | Passed the technical review and issued for public comments on August 28, 2024. |
(6) Sakura polyphenols (Draft announcement)
Name | Sakura polyphenols |
Basic information | Source: Cerasusserrulata var. lannesiana (Carr.) Makino |
Brief introduction of the production process | Made from the flowers of Japanese cherry (Prunus serrulata) as raw material, processed through ethanol extraction, filtration, concentration, drying, and grinding. |
Recommended intake | ≤ 350 mg/day (calculated as polyphenol content at 12 g/100 g, any content exceeding this value should be converted according to the actual content). |
Other information | Scope of use and maximum usage levels: - Milk and dairy products (0.4 g/kg for modified milk and flavored fermented milk, milk powder and modified milk powder calculated based on the liquid mass after reconstitution). - beverages (0.4 g/kg for liquid beverages, solid beverages calculated based on the liquid mass after reconstitution). - jelly (7 g/kg). - cocoa products, chocolate, and chocolate products (including cocoa butter substitute chocolate and its products) (7 g/kg). - confectionery (20 g/kg). - frozen desserts (4 g/kg). - baked goods (2 g/kg). - alcoholic beverages (1.5 g/kg). Infants, young children, pregnant women, and breastfeeding women are not recommended to consume this product. Labels and instructions must indicate the unsuitable groups and consumption limits. Food safety indicators shall meet the relevant requirements as detailed in the draft announcement. |
Acceptance date | Accepted on September 11, 2023. The acceptance code is衛食新進申字(2023)第0002號. |
Issued date | Passed the technical review and issued for public comments on October 24, 2024. |
(7) Rooster comb sodium hyaluronate (Draft announcement)
Name | Rooster comb sodium hyaluronate |
Basic information | Source: Rooster comb from gallus gallus domesticus |
Brief introduction of the production process | Made from rooster combs as the raw material, processed through steps such as chopping, enzymatic hydrolysis, filtration, concentration, purification, drying, and grinding. |
Recommended intake | ≤ 200 mg/day |
Other information | 1. Scope of use and maximum usage levels: - Milk and dairy products (0.2 g/kg for modified milk and flavored fermented milk, milk powder and modified milk powder calculated based on the liquid mass after reconstitution). - beverages ( liquid beverages: 2g/kg for ≤ 50 mL, 0.2 g/kg for 51-500 mL; solid beverages calculated based on the liquid mass after reconstitution). - alcoholic beverages (1 g/kg). - cocoa products, chocolate, and chocolate products (including cocoa butter substitute chocolate and its products) and confectionery (3 g/kg). - frozen desserts (2 g/kg). 2. Infants, young children, pregnant women, and breastfeeding women are not recommended to consume this product. Labels and instructions must indicate the unsuitable groups and consumption limits. 3. Food safety indicators shall meet the relevant requirements as detailed in the draft announcement. |
Acceptance date | Accepted on January 2, 2024. The acceptance code is 衛食新進申字(2024)第0001號. |
Issued date | Passed the technical review and issued for public comments on October 24, 2024. |
3. List of approved new food raw materials in 2024 (12 types)
There were 12 new food raw materials approved in 2024, including 4 strains that can be used in food, namely Pichia kluyveri, Bacillus subtilis DE111, Leuconostoc pseudomesenteroides and Bifidobacterium longum subsp. infantis M-63. The detailed approval information for each product is as follows:
(1) Dendrobium protocorm
Name | Dendrobium protocorm |
Basic information | Soure: Dendrobium officinale (Kimura et Migo) or Dendrobium huoshanense (C. Z. Tang et S. J. Cheng) |
Brief introduction of the production process | Using seeds or stems of Dendrobium officinale or Dendrobium huoshanense as raw materials, protocorms are obtained through tissue culture and then processed through collection, drying, and other steps. |
Recommended intake | Dried product: ≤ 3.5 g/day. |
1. Infants, young children, pregnant women, and breastfeeding women are not recommended to consume this product. Labels and instructions must indicate the unsuitable groups and consumption limits. 2. Food safety indicators shall meet the relevant requirements as detailed in the draft announcement. | |
Issued date | Passed the technical review and issued for public comments on December 25, 2019. |
Approved date | Officially approved on March 13, 2024 according to Notice No.2, 2024. |
(2) Meso-zeaxanthin
Name | Meso-zeaxanthin |
Basic information | Source: Tagetes erecta L. Structure:
CAS: 31272-50-1 Molecular formula: C40H56O2 Molecular weight: 568.88 |
Brief introduction of the production process | Using natural Tagetes erecta L. flowers as the raw material, processed through dehydration, crushing, extraction, isomerization, purification, drying, and other steps. |
Recommended intake | ≤8 mg/day (count as Meso-zeaxanthin) |
Other information | 1. The usage scope does not include infant food. 2. The detection method for n-hexane residue should refer to GB 24405. 3. Food safety indicators shall meet the relevant requirements as detailed in the draft announcement. |
Acceptance date | Accepted on March 17, 2022. The acceptance code is 衛食新申字(2022)第0002號. |
Issued date | Passed the technical review and issued for public comments on August 8, 2023. |
Approved date | Officially approved on March 13, 2024 according to Notice No. 2, 2024. |
(3) Pichia kluyveri
Name | Pichia kluyveri |
Other information | 1. Approved for inclusion in the List of Strains Available for Food Use, with usage limited to the fermentation processing of alcoholic beverages, fruit and vegetable juices and drinks, tea beverages, protein drinks, and plant-based drinks. Infant food is excluded. The label and instructions must clearly indicate the scope of use. 2. Food safety indicators must comply with the provisions of the National Food Safety Standard for Food Processing Strain Preparations (GB 31639-2023). |
Acceptance date | Accepted on December 5, 2022. The acceptance code is 衛食新進申字(2022)第0006號. |
Issued date | Passed the technical review and issued for public comments on August 8, 2023. |
Approved date | Officially approved on March 13, 2024 according to Notice No. 2, 2024. |
(4) Bacillus subtilis DE111
Name | Bacillus subtilis DE111 |
Other information | 1. Approved for inclusion in the List of Strains Available for Food Use. Infant food is excluded. 2. Food safety indicators must comply with the provisions of the National Food Safety Standard for Food Processing Strain Preparations (GB 31639-2023). |
Acceptance date | Accepted on March 9, 2023. The acceptance code is 衛食新申字(2023)第0003號. |
Issued date | Passed the technical review and issued for public comments on August 8, 2023. |
Approved date | Officially approved on March 13, 2024 according to Notice No. 2, 2024. |
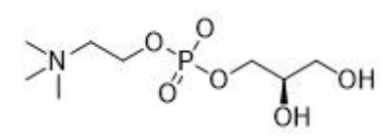
(5) L-alpha-Glycerylphosphorylcholine
Name | L-alpha-Glycerylphosphorylcholine |
Basic information | Structure:
CAS: 28319-77-9 Molecular formula: C8H20NO6P Molecular weight: 257.22 |
Brief introduction of the production process | Using polyphosphoric acid, choline chloride, R-3-chloro-1,2-propanediol, sodium hydroxide, and water as raw materials, manufactured through condensation and esterification reactions, followed by decolorization, impurity removal, concentration, refinement, and drying processes. |
Recommended intake | ≤600m g/day (count on dry basis) |
Other information | 1. Infants, young children, pregnant women, and breastfeeding women are not recommended to consume this product. Labels and instructions must indicate the unsuitable groups and consumption limits. 2. Food safety indicators shall meet the relevant requirements as detailed in the draft announcement. |
Acceptance date | Accepted on September 6, 2023. The acceptance code is 衛食新申字(2023)第0014號. |
Issued date | Passed the technical review and issued for public comments on December 22, 2023. |
Approved date | Officially approved on March 13, 2024 according to Notice No. 2, 2024 |
(6) Leuconostoc pseudomesenteroides
Name | Leuconostoc pseudomesenteroides |
Other information | 1. Approved for inclusion in the List of Strains Available for Food Use, with usage limited to the fermentation processing of alcoholic beverages, fruit and vegetable juices and drinks, tea beverages, protein drinks, and plant-based drinks. Infant food is excluded. 2. Food safety indicators must comply with the provisions of the National Food Safety Standard for Food Processing Strain Preparations (GB 31639-2023). |
Acceptance date | Accepted on September 11, 2023. The acceptance code is 衛食新進申字(2023)第0002號. |
Issued date | Passed the technical review and issued for public comments on December 22, 2023. |
Approved date | Officially approved on March 13, 2024 according to Notice No. 2, 2024 |
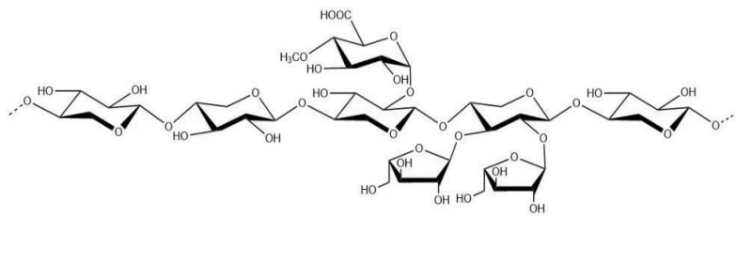
(7) Arabinoxylan
Name | Arabinoxylan |
Basic information | Structure:
CAS:9040-27-1 |
Brief introduction of the production process | Made from sugarcane bagasse as the raw material, processed through cleaning, squeezing, sodium hydroxide extraction, precipitation, purification, drying, and other steps. |
Recommended intake | ≤ 15 g/day (based on arabinoxylan content of 85 g/100 g; for contents exceeding this, the calculation should be based on the actual content). |
Other information | 1. Infants, young children, pregnant women, and breastfeeding women are not recommended to consume this product. Labels and instructions must indicate the unsuitable groups and consumption limits. 2. Food safety indicators shall meet the relevant requirements as detailed in the draft announcement. |
Acceptance date | Accepted on October 10, 2019. The acceptance code is 衛食新申字(2019)第0001號. |
Issued date | Passed the technical review and issued for public comments on March 14, 2022. |
Approved date | Officially approved on August 5, 2024 according to Notice No. 3, 2024. |
(8) Bifidobacterium longum subsp. infantis M-63
Name | Bifidobacterium longum subsp. infantis M-63 |
Other information | 1. Approved for inclusion in the List of Strains Available for Food Use. Infant food is excluded. 2. Food safety indicators must comply with the provisions of the National Food Safety Standard for Food Processing Strain Preparations (GB 31639-2023). At the same time, Cronobacter species must not be detected (/100 g). |
Acceptance date | Accepted on November 6, 2023. The acceptance code is 衛食新申字(2023)第0004號。 |
Issued date | Passed the technical review and issued for public comments on March 1, 2024. |
Approved date | Officially approved on August 5, 2024 according to Notice No. 3, 2024. |
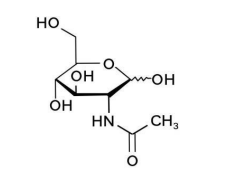
(9) N-acetylglucosamine
Name | N-acetylglucosamine |
Basic information | Structure:
CAS: 7512-17-6 Molecular formula: C8H15NO6 Molecular weight:221.21 |
Brief introduction of the production process | Made from raw materials including glucose, corn syrup powder, ammonium sulfate, potassium dihydrogen phosphate, and magnesium sulfate, processed through fermentation with Corynebacterium glutamicum RDG-2110, followed by filtration, concentration, crystallization, centrifugation, alcohol washing, drying, and other steps. |
Recommended intake | ≤ 500 mg/day (based on dry weight) |
Other information | 1. Scope of use and maximum usage levels: - Milk and dairy products (0.5 g/kg for modified milk and flavored fermented milk, milk powder and modified milk powder calculated based on the liquid mass after reconstitution). - beverages (0.5 g/kg for liquid beverages, solid beverages calculated based on the liquid mass after reconstitution). - jelly (3 g/kg). - confectionery (10 g/kg). - baked goods (2 g/kg). 2. Infants, young children, pregnant women, and breastfeeding women are not recommended to consume this product. Labels and instructions must indicate the unsuitable groups and consumption limits. 3. Food safety indicators shall meet the relevant requirements as detailed in the draft announcement. |
Acceptance date | Accepted on April 6, 2023. The acceptance code is 衛食新申字(2023)第0005號. |
Issued date | Passed the technical review and issued for public comments on March 1, 2024. |
Approved date | Officially approved on August 5, 2024 according to Notice No. 3, 2024. |
(10) Nannochloropsis gaditana oil
Name | Nannochloropsis gaditana oil |
Basic information | Source: Nannochloropsis gaditana |
Brief introduction of the production process | Made from Nannochloropsis gaditana as the raw material, processed through ethanol extraction, filtration, purification, concentration, and other steps. |
Recommended intake | ≤2 g/day |
Other information | 1. Infants, young children, pregnant women, and breastfeeding women are not recommended to consume this product. Labels and instructions must indicate the unsuitable groups and consumption limits. 2. Food safety indicators shall meet the relevant requirements as detailed in the draft announcement. |
Acceptance date | Accepted on September 4, 2023. The acceptance code is 衛食新申字(2023)第0013號. |
Issued date | Passed the technical review and issued for public comments on April 28, 2024. |
Approved date | Officially approved on October 10, 2024 according to Notice No.5, 2024. |
(11) Procyanidins
Name | Procyanidins |
Basic information | Source: barks of Pinuspinaster Aiton. |
Brief introduction of the production process | Made from the bark of Pinuspinaster Aiton, processed through grinding, ethanol extraction, filtration, concentration, drying, and other steps. |
Recommended intake | ≤ 150 mg/day (based on anthocyanin content of 50 g/100 g; for contents exceeding this, the calculation should be based on the actual content). |
Other information | 1. Scope of use and maximum usage levels: - Milk and dairy products (0.2 g/kg for modified milk and flavored fermented milk, milk powder and modified milk powder calculated based on the liquid mass after reconstitution). - beverages (0.08 g/kg for liquid beverages, solid beverages calculated based on the liquid mass after reconstitution). - jelly (1.4 g/kg). 2. Infants, young children, pregnant women, and breastfeeding women are not recommended to consume this product. Labels and instructions must indicate the unsuitable groups and consumption limits. 3. Food safety indicators shall meet the relevant requirements as detailed in the draft announcement. |
Acceptance date | Accepted on December 23, 2021. The acceptance code is 衛食新進申字(2021)第0012號. |
Issued date | Passed the technical review and issued for public comments on March 1, 2024. |
Approved date | Officially approved on October 10, 2024 according to Notice No.5, 2024. |
(12) Camellia petelotii culture
Name | Camellia petelotii culture |
Basic information | Camellia petelotii (Merrill) Sealy |
Brief introduction of the production process | Made from the leaves of Camellia petelotii (Merrill) Sealy, processed through disinfection, callus induction, cultivation, collection, drying, and grinding. |
Recommended intake | ≤1.5g/day |
Other information | 1. Infants, young children, pregnant women, and breastfeeding women are not recommended to consume this product. Labels and instructions must indicate the unsuitable groups and consumption limits. 2. Food safety indicators shall meet the relevant requirements as detailed in the draft announcement. |
Acceptance date | Accepted on January 5, 2024. The acceptance code is 衛食新申字(2024)第0003號. |
Issued date | Passed the technical review and issued for public comments on July 16, 2024. |
Approved date | Officially approved on December 13, 2024 according to Notice No.6, 2024. |
4. Raw materials included in the List of Termination of Review (9 types)
In 2024, 9 raw materials were included in the list of termination review, and currently there are 82 products on the list.
No. | Acceptance code | Name |
1 | 衛食新申字(2023)第0012號 | |
2 | 衛食新進申字(2023)第0005號 | |
3 | 衛食新申字(2024)第0004號 | |
4 | 衛食新申字(2024)第0005號 | |
5 | 衛食新申字(2022)第0015號 | |
6 | 衛食新進申字(2023)第0003號 | |
7 | 衛食新申字(2023)第0011號 | |
8 | 衛食新進申字(2021)第0006號 | |
9 | 衛食新申字(2024)第0017號 |
5. Raw materials rejected for approval in 2024 (5 types)
In 2024, 5 products were rejected for approval by NHC. Details are as follows:
No. | Acceptance date | Acceptance code | Name | Review process/conclusion |
|---|---|---|---|---|
1 | 2023-05-10 | 衛食新申字(2023)第0006號 | Pyrroloquinoline quinone disodium(PQQ)salt | 2023.06.01 Delivered a notification of review opinion on the new food raw material; 2024.07.02 Issued a decision of not granting administrative permission. |
2 | 2023-08-28 | 衛食新申字(2023)第0010號 | Dendrobium devonianum | 2023.09.26 Delivered a notification of review opinion on the new food raw material; 2024.07.02 Issued a decision of not granting administrative permission. |
3 | 2022-09-14 | 衛食新進申字(2022)第0003號 | Oriental cherry powder extract | 2022.10.18 Delivered a notification of review opinion on the new food raw material; 2024.07.02 Issued a decision of not granting administrative permission. |
4 | 2024-05-20 | 衛食新申字(2024)第0010號 | Bacillus subtilis QK02 | 2024.06.04 Delivered a notification of review opinion on the new food raw material; 2024.08.07 Issued a decision of not granting administrative permission. |
5 | 2024-05-17 | 衛食新申字(2024)第0008號 | cis-15-Tetracosenoic acid (synthetic) | 2024.06.04 Delivered a notification of review opinion on the new food raw material; 2024.12.06 Issued a decision of not granting administrative permission. |
6. Conclusion
In this year, significant progress has been made in the application of new raw materials, such as Dendrobium protocorms, arabinoxylans, and wheat polar lipids, which started their application processes earlier. The majority of raw materials submitted in the past two years have experienced an efficient process, from receiving applications to public consultations, obtaining approvals, or terminating reviews—most of which can be completed within a year, with some even within six months. This shows that the official approval process for new food ingredients has been optimized, significantly reducing evaluation times and greatly increasing the number of approvals. It also reflects the high enthusiasm from enterprises in submitting applications and the thorough preparation of documentation.
Nowadays, synthetic biology production technology has unparalleled advantages, and its application in the food sector is rapidly emerging. Against this background, on September 13, 2024, the National Center for Food Safety Risk Assessment released the Safety Evaluation Submission Requirements for Genetically Modified Microorganisms Used in Food Processing (Trial), which opens up a submission channel for new products produced by genetically engineered microorganisms. These products cover new food ingredients, new varieties of food additives, and new types of food-related products. This means that products such as allulose and N-acetylneuraminate, produced through synthetic biology, can now be submitted for approval.
Note: There may be omissions and errors in the data and statistics. The data in this article is for reference only, please refer to the official information published by government departments.
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.