On August 5, 2024, China’s National Health Commission (NHC) issued Announcement No. 3 of 2024, approving a total of eight “three new foods”, including three new food raw materials, four new food additives, and one new food-related product.
As of mid-2024, CIRS Group has made notable progress in the approval of “New Foods” in China. Five products have been officially approved, including:
- Enzymatically produced steviol glycosides: The first synthetic biology sweetener approved in China;
- Polybutylene succinate adipate (PBSA): Applicant: PTTMCC Biochem Co., Ltd., Thailand;
- Hydroxytyrosol: Applicant: Viablife;
- D-psicose 3-epimerase: Used for producing sugar substitute (D-psicose); and
- ...
In addition, six products passed the technical review by the China National Center for Food Safety Risk Assessment (CFSA) and were issued for public comment, including:
- Multiple human milk oligosaccharides (HMOs);
- Enzymes;
- Characteristic food additives; and
- Characteristic food-related products.
List of approved “three new foods”
New food raw materials (3 types)
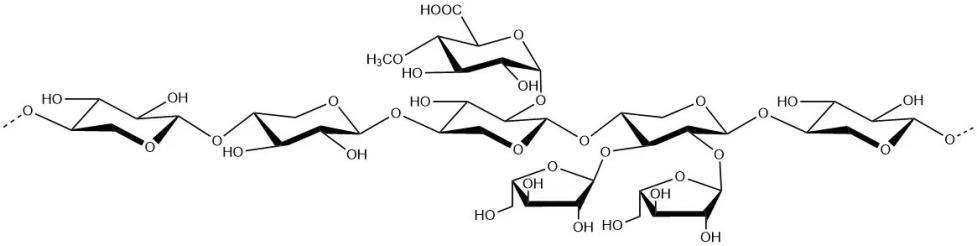
Arabinoxylan
Name | Arabinoxylan |
Basic information | Structure (fragment):
CAS number: 9040-27-1 |
Brief introduction of the production process | Prepared from sugarcane pulp through processes including washing, pressing, sodium hydroxide extraction, precipitation, purification, and drying. |
Recommended consumption level | ≤15 g/day (based on an arabinoxylan content of 85 g/100 g; amounts above this content should be adjusted according to the actual content) |
Other information | The labels and instructions must bear a statement indicating that they should not be consumed by infants, pregnant women, and breastfeeding women, along with the consumption limit. Specifications and food safety indicators are given in the Appendix. |
Bifidobacterium longum subsp. infantis M-63
Name | Bifidobacterium longum subsp. infantis M-63 |
Other information | Being included in the List of Strains that Can Be Used in Foods. Food safety indicators shall comply with the provisions of GB 31639 National Food Safety Standard - Food Processing Strains Preparation. Cronobacter should be absent/100g. |
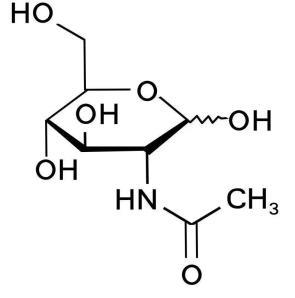
N-acetylglucosamine
Name | N-acetylglucosamine |
Basic information | Structure:
CAS number: 7512-17-6 Molecular formula: C8H15NO6 Relative molecular mass: 221.21 |
Brief introduction of the production process | Made from glucose, corn syrup powder, ammonium sulfate, potassium dihydrogen phosphate, and magnesium sulfate through processes including fermentation with Corynebacterium glutamicum RDG-2110, filtration, concentration, crystallization, centrifugation, alcohol washing, and drying. |
Recommended consumption level | ≤500 mg/day (on dry matter) |
Other information | Scope of use and maximum levels: Milk and milk products (0.5 g/kg for modified milk and flavored fermented milk, milk powder, and the formulated products are converted according to the mass of liquid after preparation); beverages (0.5 g/kg for liquid beverages, solid beverages are converted according to the mass of liquid after preparation); jelly (3 g/kg); candy (10 g/kg); pastry ( 2 g/kg); The labels and instructions must bear a statement indicating that they should not be consumed by infants, pregnant women, and breastfeeding women, along with the consumption limit. Specifications and food safety indicators are given in the Appendix. |
New food additives (four types)
1. New food additives
Hydroxytyrosol
Maximum level and scope of use:
No. | Food additive | Function | Food category number | Food name/category | Maximum level (g/kg) | Note |
1 | Hydroxytyrosol | Antioxidant | 02.01.01 | Vegetable oils and fats | 0.05 | — |
Specifications: The specifications apply to the food additive hydroxytyrosol, which is produced from tyrosine through a process involving fermentation with Corynebacterium glutamicum, followed by catalytic conversion, separation, and purification.
Methylene chloride
Maximum level and scope of use:
No. | Processing aid | Function | Scope of use |
1 | Methylene chloride | Extracting solvent | Tea decaffeination process (residue ≤ 2 mg/kg) |
Specifications: The specifications apply to the food additive methylene chloride, which is produced from methanol and hydrochloric acid. The process involves catalysis with zinc chloride to generate chloromethane, which is then reacted with chlorine to form various chlorinated methane compounds. These are subsequently purified by distillation to obtain the final product.
2. New food nutrition enhancer
2’-fucosyllactose, 2’-FL
The scope of use, maximum levels, and specifications for 2’-fucosyllactose shall comply with Announcement No. 8 of 2023 issued by the NHC (excluding Appendix C – Information on the production strain for producing 2’-fucosyllactose). The details of the production strain are as follows:
Table 1 Information on the production strain for producing 2’-fucosyllactose
Nutrition enhancer | Source | Donor |
2’-fucosyllactose | Escherichia coli BL21 star (DE3) | Escherichia coli O126a |
Corynebacterium glutamicum ATCC 13032 | Pseudopedobacter saltansa |
a is the donor for α-1,2-fucosyltransferase
3. Food additive with expanded scope of use
No. | Food additive | Function | Food category number | Food name/category | Maximum level (g/kg) | Note |
1 | Polyglycerol polyricinoleate | Emulsifier | 01.05.03 | Formulated cream | 10.0 | — |
New food-related product (one type)
New food contact resin
Poly (neopentyl glycol-co-terephtalicacid-co-monoethylene glycol-co-isophtalicacid-co-monopropylene glycol-co-Fattyacids, C18-unsaturated, dimers,hydrogenated-co-1,6-haxanediolco-trimethylol propane)
Name | Poly (neopentyl glycol-co-terephtalicacid-co-monoethylene glycol-co-isophtalicacid-co-monopropylene glycol-co-Fattyacids, C18-unsaturated, dimers,hydrogenated-co-1,6-haxanediolco-trimethylol propane) |
CAS number | — |
Scope of use | Coatings and coating film |
Maximum level/% | 69 (counted as coatings formula) |
SML (mg/kg) | 0.05 (2,2-Dimethyl-1,3-propanediol); 7.5 (count as terephthalic acid); 30 (count as ethylene glycol); 5 (count as isophthalic acid); 0.05 (count as 1,6-Hexanediol); 6 (Trimethylolpropane) |
QM (mg/kg) | — |
Note | The substance is not permitted for use in producing food contact materials and articles for infants and young children. These restrictions must be indicated in accordance with the provisions of GB 4806.1. |
About the CIRS Food Team
Our food team offers comprehensive global food compliance services, including:
New ingredients registration services:
- New food ingredients registration in China;
- New food additives registration in China;
- New food contact additives and resins registration;
- US FDA GRAS certification;
- US NDI certification;
- EU Novel Food certification;
- EU food additives and enzymes certification;
- Novel Food certification in Australia and New Zealand; and
- Feed and feed additives application in China, Europe, and the US.
Finished product compliance services in China
- Health food registration/filing;
- New health functions application;
- SAMR infant formula milk powder registration;
- SAMR food for special medical purpose (FSMP) registration;
- Label translation and review of pre-packaged general food; and
- GACC Overseas Manufacturers Registration of Imported Food (GACC Decree No. 248).
US FDA certification:
- US FDA registration of food facilities;
- US FDA food/dietary supplement ingredient and label review;
- US FDA dietary supplement structure/function claim notification; and
- Amazon product page review service.
Contact Us
Tel: +86 571 8720 6538
Email: service@cirs-group.com
Further Information