As of the end of December 2023, the National Health Commission of the People’s Republic of China (NHC) issued five announcements (No. 1 of 2023, No. 3 of 2023, No. 5 of 2023, No. 8 of 2023, and No. 10 of 2023) regarding “Three New Foods”. A total of 74 products have been approved, 33 of which are new food additives, including those with expanded scope.
Related Links
Analysis on the Application and Approval of New Food Raw Materials (Novel Food) in 2023
CIRS Group has summarized the acceptance and approval status of new food additives in China in 2023 as follows:
Overview of new food additives accepted, issued for public comments and approved in 2023
In 2023, NHC accepted the application of 82 new food additives, including both new types and those with expanded use and scope. The China National Center for Food Safety Risk Assessment (CFSA) issued a total of 22 new food additives for public comment; moreover, HNC approved 33 new food additives, including the ones with expanded scope.
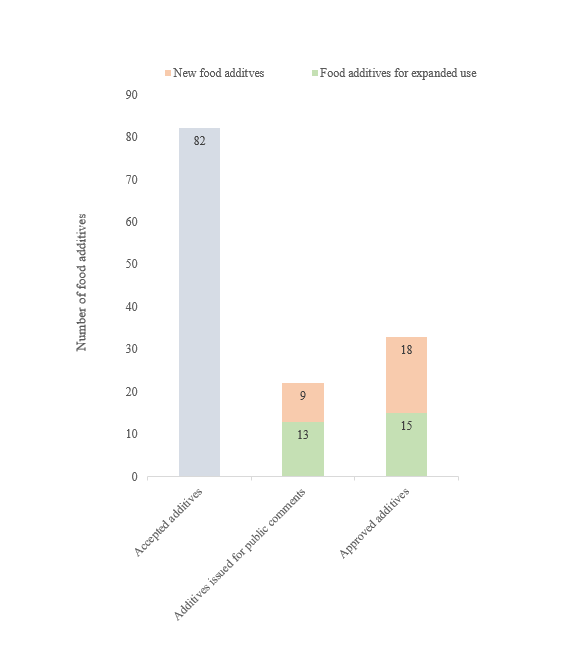
Figure 1. Overview of new food additives application in 2023
New food additives accepted by NHC in 2023 (82 types)
In 2023, NHC accepted a total of 82 new food additives, including those with expanded scope, with the acceptance codes of both domestic and imported products starting with “衛食添新申字”. Detailed information is shown in the Table below:
No. | Acceptance date | Acceptance code | Name of new food additives |
|---|---|---|---|
1 | 2023.01.03 | 衛食添新申字(2023)第0001號 | Phosphoric acid (Wet process) |
2 | 2023.01.05 | 衛食添新申字(2023)第0002號 | Calcium lactate |
3 | 2023.01.05 | 衛食添新申字(2023)第0003號 | 2’-fucosyllactose, 2’-FL |
4 | 2023.01.10 | 衛食添新申字(2023)第0004號 | Calcium chloride |
5 | 2023.01.10 | 衛食添新申字(2023)第0005號 | Sodium metabisulphite |
6 | 2023.01.10 | 衛食添新申字(2023)第0006號 | Benzoic acid, sodium benzoate |
7 | 2023.01.10 | 衛食添新申字(2023)第0007號 | Sorbic acid, potassium sorbate |
8 | 2023.01.10 | 衛食添新申字(2023)第0008號 | Ascorbic acid |
9 | 2023.01.10 | 衛食添新申字(2023)第0009號 | Paprika red |
10 | 2023.01.10 | 衛食添新申字(2023)第0010號 | Lactic acid |
11 | 2023.01.10 | 衛食添新申字(2023)第0011號 | Acesulfame potassium |
12 | 2023.01.10 | 衛食添新申字(2023)第0012號 | Paprika oleoresin |
13 | 2023.01.10 | 衛食添新申字(2023)第0013號 | Sucralose |
14 | 2023.01.10 | 衛食添新申字(2023)第0014號 | Tartrazine |
15 | 2023.01.10 | 衛食添新申字(2023)第0015號 | D-isoascorbic acid (erythorbic acid) , sodium D-isoascorbate |
16 | 2023.01.10 | 衛食添新申字(2023)第0016號 | Disodium 5'-ribonucleotide |
17 | 2023.01.10 | 衛食添新申字(2023)第0017號 | Citric acid |
18 | 2023.01.10 | 衛食添新申字(2023)第0018號 | Dehydroacetic acid, sodium dehydroacetate |
19 | 2023.01.10 | 衛食添新申字(2023)第0019號 | Disodium ethylene-diamine-tetra-acetate |
20 | 2023.01.10 | 衛食添新申字(2023)第0020號 | Hydroxytyrosol |
21 | 2023.01.12 | 衛食添新申字(2023)第0021號 | Nitrous oxide |
22 | 2023.01.28 | 衛食添新申字(2023)第0022號 | NMN (β-Nicotinamide mononucleotide) |
23 | 2023.01.29 | 衛食添新申字(2023)第0023號 | Propylene glycol alginate |
24 | 2023.02.02 | 衛食添新申字(2023)第0024號 | D-3-hydroxybutyric acid |
25 | 2023.02.17 | 衛食添新申字(2023)第0025號 | Runmai improver |
26 | 2023.02.27 | 衛食添新申字(2023)第0026號 | Carmine cochineal |
27 | 2023.03.03 | 衛食添新申字(2023)第0027號 | Vitamin K2 (synthesis method) |
28 | 2023.03.03 | 衛食添新申字(2023)第0028號 | Cyclomaltodextin glucanotransferase |
29 | 2023.03.06 | 衛食添新申字(2023)第0029號 | Edible tannin |
30 | 2023.03.06 | 衛食添新申字(2023)第0030號 | Rebaudioside M |
31 | 2023.03.30 | 衛食添新申字(2023)第0031號 | Corderan gum |
32 | 2023.04.23 | 衛食添新申字(2023)第0032號 | D-psicose 3-epimerase |
33 | 2023.05.04 | 衛食添新申字(2023)第0033號 | 6’-sialyllactose sodium salt, 6’-SL |
34 | 2023.05.10 | 衛食添新申字(2023)第0034號 | Rebaudioside M |
35 | 2023.05.22 | 衛食添新申字(2023)第0035號 | Xylanase |
36 | 2023.05.25 | 衛食添新申字(2023)第0036號 | Sodium sulfide pentahydrate |
37 | 2023.05.31 | 衛食添新申字(2023)第0037號 | Propylene glycol alginate |
38 | 2023.06.02 | 衛食添新申字(2023)第0038號 | Vitamine E (dl-α-tocopherol, d-α-tocopherol, mixed tocopherol concentrate) |
39 | 2023.07.03 | 衛食添新申字(2023)第0039號 | L-alanine |
40 | 2023.07.04 | 衛食添新申字(2023)第0040號 | Ramie Leaf Extract |
41 | 2023.07.04 | 衛食添新申字(2023)第0041號 | Stevia glycoside |
42 | 2023.07.05 | 衛食添新申字(2023)第0042號 | Tetrapotassium pyrophosphate |
43 | 2023.07.06 | 衛食添新申字(2023)第0043號 | Sucralose |
44 | 2023.07.13 | 衛食添新申字(2023)第0044號 | Nitric acid |
45 | 2023.07.14 | 衛食添新申字(2023)第0045號 | 2’-fucosyllactose, 2’-FL |
46 | 2023.07.17 | 衛食添新申字(2023)第0046號 | 6’-Sialyllactose sodium salt, 6’-SL |
47 | 2023.07.17 | 衛食添新申字(2023)第0047號 | 3’-Sialyllactose sodium salt, 3’-SL |
48 | 2023.08.01 | 衛食添新申字(2023)第0048號 | Potassium sorbate |
49 | 2023.08.11 | 衛食添新申字(2023)第0049號 | Polyoxyethylene (20) sorbitan monooleat |
50 | 2023.09.04 | 衛食添新申字(2023)第0050號 | Lycopene |
51 | 2023.09.06 | 衛食添新申字(2023)第0051號 | (6S)-5-methyltetrahydrofolic acid, glucosamine salt |
52 | 2023.09.06 | 衛食添新申字(2023)第0052號 | Butylated hydroxytoluene (BHT) |
53 | 2023.09.07 | 衛食添新申字(2023)第0053號 | Rosemary extract |
54 | 2023.09.15 | 衛食添新申字(2023)第0054號 | Hydroxytyrosol |
55 | 2023.09.18 | 衛食添新申字(2023)第0055號 | Sanzan Gum |
56 | 2023.09.18 | 衛食添新申字(2023)第0056號 | Sanzan Gum |
57 | 2023.09.18 | 衛食添新申字(2023)第0057號 | Lactase (beta-galactosidase) |
58 | 2023.09.19 | 衛食添新申字(2023)第0058號 | 2’-fucosyllactose, 2’-FL |
59 | 2023.09.20 | 衛食添新申字(2023)第0059號 | 2’-fucosyllactose, 2’-FL |
60 | 2023.09.20 | 衛食添新申字(2023)第0060號 | Nitrous oxide |
61 | 2023.09.20 | 衛食添新申字(2023)第0061號 | 2’-fucosyllactose, 2’-FL |
62 | 2023.09.21 | 衛食添新申字(2023)第0062號 | 3’-Sialyllactose sodium salt, 3’-SL |
63 | 2023.09.22 | 衛食添新申字(2023)第0063號 | Phosphodiesterase I |
64 | 2023.11.01 | 衛食添新申字(2023)第0064號 | 2’-fucosyllactose, 2’-FL |
65 | 2023.11.01 | 衛食添新申字(2023)第0065號 | 2’-fucosyllactose, 2’-FL |
66 | 2023.11.03 | 衛食添新申字(2023)第0066號 | Glucoamylase |
67 | 2023.11.03 | 衛食添新申字(2023)第0067號 | Serine protease |
68 | 2023.11.06 | 衛食添新申字(2023)第0068號 | Tripotassium citrate |
69 | 2023.11.08 | 衛食添新申字(2023)第0069號 | Ascorbyl palmitate (enzymatic) |
70 | 2023.11.13 | 衛食添新申字(2023)第0070號 | Zinc sulfate |
71 | 2023.11.20 | 衛食添新申字(2023)第0071號 | Lactase (beta-galactosidase) |
72 | 2023.11.20 | 衛食添新申字(2023)第0072號 | Lipase |
73 | 2023.11.20 | 衛食添新申字(2023)第0073號 | Calcium carbonate (seaweed source) |
74 | 2023.11.21 | 衛食添新申字(2023)第0074號 | 3-fucosyllactose, 3-FL |
75 | 2023.11.21 | 衛食添新申字(2023)第0075號 | Sodium sulfide pentahydrate |
76 | 2023.11.30 | 衛食添新申字(2023)第0076號 | 2’-fucosyllactose, 2’-FL |
77 | 2023.11.30 | 衛食添新申字(2023)第0077號 | Cellulose |
78 | 2023.12.08 | 衛食添新申字(2023)第0078號 | Sanzan Gum |
79 | 2023.12.11 | 衛食添新申字(2023)第0079號 | Selenium-enriched yeast |
80 | 2023.12.25 | 衛食添新申字(2023)第0080號 | Pomelone [(8E) -undecan-6,8, 10-triene-3-ketone] |
81 | 2023.12.25 | 衛食添新申字(2023)第0081號 | (E)-6-Octenal |
82 | 2023.12.29 | 衛食添新申字(2023)第0082號 | Sanzan Gum |
Note: Substances in blue have been issued a rejection decision.
New food additives that have passed the technical review and were issued for public comments in 2023 (22 types)
As of the end of December 2023, 22 new food additives have passed the technical review by the review committee of China CFSA and were issued for public comments, including 6 new food enzymes, 2 new food additives, 1 new nutrition enhancer, 10 food additives with expanded use and 3 food processing aids with expanded use.
(1) New food enzymes issued for public comments (6 types)
S.N. | Name | Source | Donor | Approval status (As of the end of December 2023) |
|---|---|---|---|---|
1 | Beta-amylase | Bacillus flexus | — | 1) Accepted on July 7, 2023. Acceptance code: 衛食添新申字(2021)第0029號; 2) Issued for public comments on February 10, 2023; 3) Officially approved on August 1, 2023 according to Notice No. 5 of 2023. |
2 | Lysophospholipase (lecithinase B) | Trichoderma reesei | Aspergillus nishimurae | 1) Accepted on July 15, 2022. Acceptance code: 衛食添新申字(2022)第0063號; 2) Issued for public comments on February 10, 2023; 3) Officially approved on August 1, 2023 according to Notice No. 5 of 2023. |
3 | Serine protease | Bacillus licheniformis | Nocardiopsis prasina | 1) Accepted on May 10, 2022. Acceptance code: 衛食添新申字(2022)第0018號; 2) Issued for public comments on April 24, 2023; 3) Officially approved on October 7, 2023 according to Notice No. 8 of 2023. |
4 | Cyclomaltodextin glucanotransferase | Anoxybacillus caldiproteolyticus | — | 1) Accepted on March 3, 2023. Acceptance code: 衛食添新申字(2023)第0028號; 2) Issued for public comments on October 26, 2023; 3) It has not been officially approved yet. |
5 | Cellulase | Penicillium oxalicum | — | 1) Accepted on September 14, 2022. Acceptance code: 衛食添新申字(2022)第0072號; 2) Issued for public comments on October 26, 2023; 3) It has not been officially approved yet. |
6 | D-psicose 3-epimerase | Bacillus subtilis | Clostridium scindens ATCC35704 | 1) Accepted on April 23, 2023. Acceptance code: 衛食添新申字(2023)第0032號; 2) Issued for public comments on December 29, 2023; 3) It has not been officially approved yet. |
(2) New food additives issued for public comments (2 types)
S.N. | Name | Function | Category number | Food name/ category | Maximum levels (g/kg) | Approval status (As of the end of December 2023) |
1 | Mixed tocotrienol concentrate | Antioxidant | 02.01.01 | Vegetable oils and fats | 0.2 (counted as the total amount of tocopherol and tocotrienol) | 1) Accepted on June 21, 2019. Acceptance code: 衛食添新申字(2019)第0037號; 2) Issued for public comments on June 26, 2023; 3) It has not been officially approved. |
2 | Rebaudioside M | Sweetener | 01.01.03 | Modified milk | 0.18 (count as steviols) | 1) Accepted on May 10, 2023. Acceptance code: 衛食添新申字(2023)第0034號; 2) Issued for public comments on October 26, 2023; 3) It has not been officially approved yet. |
01.02.02 | Flavored fermented milk | 0.2 (count as steviols) | ||||
03.01 | Ice cream and iced milk | 0.5 (count as steviols) | ||||
05.02.01 | Gum-based candy | 3.5 (count as steviols) | ||||
14.0 | Beverages (except 14.01 packaged drinking water) | 0.2 (count as steviols; count as the state of ready-to-drink, increase the usage of solid drinks according to the dilution ratio.) |
(3) New nutrition enhancers issued for public comments (1 type)
No. | Name | Applicable scope | Maximum levels | Production strain information | Approval status (By the end of December 2023) |
|---|---|---|---|---|---|
1 | 2’-fucosyllactose | 01.03.02, modified milk powder (for children only); 13.01.01, infant formula food; 13.01.02, formula for older infants and young children; 13.01.03, infant formula food for special medical purpose | 0.7-2.4 g/L (count as the state of ready-to-eat; for powdery products, the level of use should be increased by times of brewing); when mixed with Lacto-N-neotetraose (LNnT), galacto-oligosaccharide (GOS), fructo-oligosaccharide (FOS), polyfructose, and raffinose, the total amount of 2’-fucosyllactose shall not exceed 64.5 (g/kg). | Source: E. coli BL21(DE3); Donor: Neisseria spp. | 1) Accepted on January 5, 2023. Acceptance code: 衛食添新申字(2023)第0003號; 2) Issued for public comments on February 10, 2023; 3) Officially approved on October 7, 2023 according to Notice No. 8 of 2023. |
Source: Corynebacterium glutamate ATCC 13032; Donor: Pseudopedobacter saltans | 1) Accepted on July 14, 2023. Acceptance code: 衛食添新申字(2023)第0045號; 2) Issued for public comments on August 23, 2023; 3) It has not been officially approved yet. | ||||
Source: Escherichia coli BL21(DE3); Donor: Helicobacter pylori | 1) Accepted on September 20, 2023. Acceptance code: 衛食添新申字(2023)第0061號; 2) Issued for public comments on October 26, 2023; 3) It has not been officially approved yet. |
(4) Food additives with expanded scope issued for public comments (10 types)
S.N. | Name | Function | Category number | Food name/ category | Maximum levels (g/kg) | Remarks | Approval status (By the end of December 2023) |
|---|---|---|---|---|---|---|---|
1 | Calcium lactate | Stabilizer, coagulant, and acidity regulator | 04.02.02.03 | Pickled vegetables | 10.0 | - | 1) Accepted on January 5, 2023. Acceptance code: 衛食添新申字(2023)第0002號; 2) Issued for public comments on April 24, 2023; 3) Officially approved on October 7, 2023 according to Notice No. 8 of 2023. |
04.02.02.04 | Canned vegetables | 3.0 | - | ||||
2 | Sanzan Gum | Thickener, stabilizer and coagulant | 01.01.03 | Modified milk | 0.5 | - | 1) No acceptance information was found; 2) Issued for public comments on February 10, 2023; 3) Officially approved on October 7, 2023 according to Notice No. 8 of 2023. |
03.01 | Ice cream and iced milk | 1.5 | - | ||||
14.03.03 | Mixed protein drinks | 0.75 | Count as the state of ready-to-drink, increase the usage of solid drinks according to the dilution ratio. | ||||
14.08 | Flavored drinks | 0.5 | |||||
01.01.03 | Modified milk | 0.5 | - | 1) No acceptance information was found; 2) Issued for public comments on April 24, 2023; 3) Officially approved on October 7, 2023 according to Notice No. 8 of 2023. | |||
14.03.03 | Mixed protein drinks | 0.75 | Count as the state of ready-to-drink, increase the usage of solid drinks according to the dilution ratio. | ||||
14.08 | Flavored drinks | 0.5 | |||||
3 | L-alanine | Flavor enhancer | 14.02.03 | Fruit and vegetable juice (pulp) drinks | 6.0 | Count as the state of ready-to-drink, increase the usage of solid drinks according to the dilution ratio. | 1) Accepted on July 3, 2023. Acceptance code:衛食添新申字(2023)第0039號; 2) Issued for public comments on August 23, 2023; 3) It has not been officially approved yet. |
4 | Propylene glycol alginate | Thickener | 06.05.02.01 | Bean vermicelli, bean noodles | 1.5 | - | 1) Accepted on May 31, 2023. Acceptance code: 衛食添新申字(2023)第0037號; 2) Issued for public comments on August 23, 2023; 3) It has not been officially approved yet. |
06.05.02.04 | Round rice ball | ||||||
5 | Lycopene | Colorant | 08.02.01 | Seasoned meat products (adding seasonings to fresh meats) | 0.12 | Count as lycopene | 1) Accepted on September 4, 2023. Acceptance code: 衛食添新申字(2023)第0050號; 2) Issued for public comments on October 26, 2023; 3) It has not been officially approved yet. |
08.02.02 | Cured meat products (e.g., salt meats, cured meats, dried salted duck, Chinese ham and sausage) | 0.018 | |||||
08.03.05 | Sausage | 0.35 | |||||
08.03.07. 03 | Dried meat crisps | 0.26 | |||||
6 | Polyoxyethylene (20) sorbitan monooleat | Emulsifier | 16.03 | Collagen casing | 0.5 | - | 1) Accepted on August 11, 2023. Acceptance code: 衛食添新申字(2023)第0049號; 2) Issued for public comments on October 26, 2023; 3) It has not been officially approved yet. |
7 | Rosemary extract | Antioxidant | 04.05.02 | Processed nuts and seeds | 0.3 | - | 1) Accepted on September 7, 2023. Acceptance code: 衛食添新申字(2023)第0053號; 2) Issued for public comments on October 26, 2023; 3) It has not been officially approved yet. |
8 | Vitamine E (dl-α-tocopherol, d-α-tocopherol, mixed tocopherol concentrate) | Antioxidant | 16.07 | Others (Lutein Esters only) | 50 | - | 1) Accepted on June 2, 2023. Acceptance code: 衛食添新申字(2023)第0038號; 2) Issued for public comments on October 26, 2023; 3) It has not been officially approved yet. |
9 | Ascorbyl palmitate (enzymatic) | Antioxidant | 01.03 | Milk powder (including sweetened milk powder) , cream powder and its formulated products | 0.2 | Count as ascorbic acid in fat | 1) Accepted on November 8, 2023. Acceptance code: 衛食添新申字(2023)第0069號; 2) Issued for public comments on December 29, 2023; 3) It has not been officially approved yet. |
07.01 | Bread | 0.2 | |||||
14.05.01 | Tea drinks | 0.2 | Count as the state of ready-to-drink, increase the usage of solid drinks according to the dilution ratio. | ||||
10 | Sucralose | Sweetener | 04.05.02.01.01 | Shelled and cooked processed nuts and seeds | 4.0 | - | 1) Accepted on January 10, 2023. Acceptance code: 衛食添新申字(2023)第0013號; 2) Issued for public comments on December 29, 2023; 3) It has not been officially approved yet. |
04.05.02.01.02 | Unshelled and cooked processed nuts and seeds | 2.0 |
(5) Food processing aids with expanded scope issued for public comments (3 types)
S.N. | Name | Function | Applicable scope | Approval status (By the end of December 2023) |
|---|---|---|---|---|
1 | Sulfuric acid | Neutralizing and removing soap | Processing technology of oil | 1) Accepted on December 19, 2022. Acceptance code: 食添新申字(2022)第0095號; 2) Issued for public comments on February 10, 2023; 3) Officially approved on August 1, 2023 according to Notice No. 5 of 2023. |
2 | Edible tannin | clarifying agent | Sugar technology | 1) Accepted on March 6, 2023. Acceptance code: 衛食添新申字(2023)第0029號; 2) Issued for public comments on June 26, 2023; 3) Officially approved on December 1, 2023 according to Notice No. 10 of 2023. |
3 | Ethyl acetate | Extracting solvent | Processing technology of tea extracts | 1) Accepted on November 11, 2022. Acceptance code: 衛食添新申字(2022)第0083號; 2) Issued for public comments on June 26, 2023; 3) Officially approved on December 1, 2023 according to Notice No. 10 of 2023. |
Approved new food additives in 2023 (33 types)
In 2023, 33 new food additives were approved by NHC, including 18 new food additives, and 15 food additives with expanded use and scope. Notably, ascorbyl palmitate (enzymatic) was approved as both a food additive and a nutrition enhancer with expanded use. Detailed information is as follows:
(1) Approved new food enzymes (12 types)
S.N. | Name | Source | Donor | Approval status (As of the end of December 2023) |
|---|---|---|---|---|
1 | Aminopeptidase | Aspergillus oryzae | Aspergillus oryzae | 1) Accepted on March 7, 2022. Acceptance code: 衛食添新申字(2022)第0010號; 2) Issued for public comments on July 1, 2022; 3) Officially approved on March 2, 2023 according to No. 1 of 2023. |
2 | Protease | Trichoderma reesei | Malbranchea sulfurea | 1) No acceptance information found; 2) Issued for public comments on October 28, 2022; 3) Officially approved on March 2, 2023 according to No. 1 of 2023. |
3 | Phospholipase A2 | Trichoderma reesei | Aspergillus fumigatus | 1) Accepted on April 28, 2022. Acceptance code: 衛食添新申字(2022)第0017號; 2) Issued for public comments on October 28, 2022; 3) Officially approved on March 2, 2023 according to No. 1 of 2023. |
4 | Maltogenic amylase | Saccharomyces cerevisiae | Geobacillus stearothermophilus | 1) Accepted on March 19, 2021. Acceptance code:衛食添新申字(2021)第0014號; 2) Issued for public comments on August 6, 2021; 3) Officially approved on March 2, 2023 according to No. 1 of 2023. |
5 | Xylanase | Bacillus licheniformis | Bacillus licheniformis | 1) Accepted on March 7, 2022. Acceptance code: 衛食添新申字(2022)第0009號; 2) Issued for public comments on July 1, 2022; 3) Officially approved on March 2, 2023 according to No. 1 of 2023. |
6 | Lactase (beta-galactosidase) | Papiliotrema terrestris | - | 1) Accepted on April 26, 2022. Acceptance code: 衛食添新申字(2022)第0016號; 2) Issued for public comments on October 28, 2022; 3) Officially approved on March 2, 2023 according to No. 1 of 2023. |
7 | Carboxypeptidase | Aspergillus oryzae | Aspergillus oryza | 1) Accepted on January 6, 2022. Acceptance code: 衛食添新申字(2022)第0001號; 2) Issued for public comments on July 1, 2022; 3) Officially approved on March 2, 2023 according to No. 1 of 2023. |
8 | Deaminase | Aspergillus oryzae | - | 1) Accepted on April 30, 2020. Acceptance code: 衛食添新申字(2020)第0020; 2) Issued for public comments on July 1, 2022; 3) Officially approved on March 2, 2023 according to No. 1 of 2023. |
9 | D-psicose 3-epimerase | Bacillus subtilis | Ruminococcus sp. CAG55 | 1) Accepted on June 2, 2022. Acceptance code: 衛食添新申字(2022)第0033號; 2) Issued for public comments on December 8, 2022; 3) Officially approved on May 6, 2023 according to No. 3 of 2023. |
10 | Beta-amylase | Bacillus flexus | — | 1) Accepted on July 7, 2021. Acceptance code: 衛食添新申字(2021)第0029號; 2) Issued for public comments on February 10, 2023; 3) Officially approved on August 1, 2023 according to No. 5 of 2023. |
11 | Lysophospholipase (lecithinase B) | Trichoderma reesei | Aspergillus nishimura | 1) Accepted on July 15, 2022. Acceptance code: 衛食添新申字(2022)第0063號; 2) Issued for public comments on February 10, 2023. 3) Officially approved on August 1, 2023 according to No. 5 of 2023. |
12 | Serine proteas | Bacillus licheniformis | Nocardiopsis prasina | 1) Accepted on May 10, 2022. Acceptance code: 衛食添新申字(2022)第0018號; 2) Issued for public comments on April 24, 2023; 3) Officially approved on October 7, 2023 according to No. 8 of 2023. |
Note: The quality specifications of food enzymes shall meet the requirements specified in the National Food Safety Standard - Food Additives and Enzymes (GB 1886.174).
(2) Approved new food additives (1 type)
S.N. | Name | Function | Category number | Food name/ category | Maximum levels (g/L) | Acceptance information, issuance for public comments and approval (As of the end of December 2023) |
1 | Potassium Polyaspartate | Stabilizer and coagulant | 15.03.01 | Grape wine | 0.3 | 1) Accepted on May 9, 2020. Acceptance code: 衛食添新申字(2020)第0024號; 2) Issued for public comments on October 28, 2022. 3) Officially approved on March 2, 2023 according to No. 1 of 2023. |
(3) New spice for food (1 type)
S.N. | Name | Function | Category number | Food name/ category | Maximum level (g/L) | Acceptance information, issuance for public comments and approval (As of the end of December 2023) |
1 | 2-Hexylpyridine | Spice for food | - | Prepared as spices food for use in various types of food (excluding food categories listed in Table B.1 of GB 2760-2014) | Appropriate level | 1) Accepted on May 23, 2022. Acceptance code: 衛食添新申字(2022)第0021號; 2) Issued for public comments on October 28, 2022. 3) Officially approved on March 2, 2023 according to No. 1 of 2023. |
(4) New food nutrition enhancers (4 types)
S.N. | Name | Applicable scope | Use levels | Information on producing bacteria | Acceptance information, issuance for public comments and approval (As of the end of December 2023) |
|---|---|---|---|---|---|
1 | L-Se-methylselenocysteine | The scope of use and dosage of L-Se-methylselenocysteine shall comply with the requirements for selenium as specified in the National Food Safety Standard for the Use of Food Nutrition Enhancer (GB14880) | 1) Accepted on June 13, 2022. Acceptance code: 衛食添新申字(2022)第0035號; 2) Issued for public comments on August 19, 2022; 3) Officially approved on May 6, 2023 according to No. 3 of 2023. | ||
2 | Magnesium lactate | The scope of use and dosage of magnesium lactate shall comply with the requirements for magnesium as specified in the National Food Safety Standard for the Use of Food Nutrition Enhancer (GB14880) | 1) Accepted on June 20, 2022. Acceptance code: 衛食添新申字(2022)第0038號; 2) Issued for public comments on December 8, 2022; 3) Officially approved on October 7, 2023 according to No. 8 of 2023. | ||
3 | 2’-fucosyllactose, 2’-FL | 01.03.02, modified milk powder (for children only); 13.01.01, infant formula food; 13.01.02, formula for older infants and young children; 13.01.03, infant formula food for special medical purpose | 0.7-2.4 g/L (count as the state of ready-to-eat; for powdery products, the level of use should be increased by times of brewing); when mixed with Lacto-N-neotetraose (LNnT), galacto-oligosaccharide (GOS), fructo-oligosaccharide (FOS), polyfructose and raffinose, the total amount of 2’-fucosyllactose shall not exceed 64.5 (g/kg). | Source: E. coli K-12 DH1 MDO Donor: Helicobacter spp. | 1) Accepted on October 18, 2021, and November 3, 2021, respectively. Acceptance code: 衛食添新申字(2021)第0048號 and 衛食添新申字(2021)第0050號; 2) Issued for public comments, on April 15, 2022 and October 28, 2022, respectively; 3) Officially approved on October 7, 2023 according to No. 8 of 2023. |
Source: E. coli K-12 MG1655 Donor: Helicobacter spp. | |||||
Source: E. coli BL21(DE3) Donor: Neisseria spp. | 1) Accepted on January 5, 2023. Acceptance code: 衛食添新申字(2023)第0003號; 2) Issued for public comments on February 10, 2023. 3) Officially approved on October 7, 2023 according to No. 8 of 2023. | ||||
4 | Lacto-N-neotetraose, LNnT | 01.03.02, modified milk powder (for children only); 13.01.01, infant formula food; 13.01.02, formula for older infants and young children; 13.01.03, infant formula food for special medical purpose | 0.2-0.6 g/L (count as the state of ready-to-eat; for powdery products, the level of use should be increased by times of brewing); when mixed with 2’-fucosyllactose, galacto-oligosaccharide (GOS), fructo-oligosaccharide (FOS), polyfructose and raffinose, the total amount shall not exceed 64.5 (g/kg). | Source: E. coli K-12 DH1 MDO Donor: Neisseria spp. and Helicobacter spp. | 1) Accepted on October 18, 2021. Acceptance code: 衛食添新申字(2021)第0049號; 2) Issued for public comments on October 28, 2022; 3) Officially approved on October 7, 2023 according to No. 8 of 2023. |
(5) Approved new food additives with expanded scope and use (7 types)
S.N. | Name | Function | Category number | Food name/category | Maximum levels (g/kg) | Acceptance information, issuance for public comments and approval (As of the end of December 2023) | ||
|---|---|---|---|---|---|---|---|---|
1 | Fumaric acid | Acidity regulator | 08.02.02 | Cured meat products (e.g., salt meats, cured meats, dried salted duck, Chinese ham and sausage) | Appropriate level | 1) Accepted on June 28, 2022. Acceptance code: 衛食添新申字(2022)第0040號; 2) Issued for public comments on August 19, 2022; 3) Officially approved on March 2, 2023 according to No. 1 of 2023. | ||
08.03.02 | Smoked, barbecued, and roasted meats | |||||||
08.03.03 | Fried meats | |||||||
08.03.05 | Sausages | |||||||
09.02.02 | Frozen coating products | |||||||
09.04.02 | Cooked or fired aquatic products | |||||||
09.04.03 | Smoked, baked aquatic products | |||||||
2 | Sodium acetate | Acidity regulator | 08.02.02 | Cured meat products (e.g., salt meats, cured meats, dried salted duck, Chinese ham and sausage) | Appropriate level | 1) Accepted on June 28, 2022. Acceptance code: 衛食添新申字(2022)第0039號; 2) Issued for public comments on August 19, 2022; 3) Officially approved on March 2, 2023 according to No. 1 of 2023. | ||
08.03.02 | Smoked, barbecued, and roasted meats | |||||||
08.03.03 | Fried meats | |||||||
08.03.05 | Sausages | |||||||
09.02.02 | Frozen coating products | |||||||
09.04.02 | Cooked or fired aquatic products | |||||||
09.04.03 | Smoked, baked aquatic products | |||||||
3 | Sodium cyclamate, calcium cyclamate | Sweetener | 07.04 | Fillings and surface spreading starch for bakery food (for bakery food fillings only) | 2.0 | Count as cyclamic acid | 1) Accepted on July 12, 2022. Acceptance code: 衛食添新申字(2022)第0060號; 2) Issued for public comments on October 28, 2022; 3) Officially approved on March 2, 2023 according to No. 1 of 2023. | |
16.06 | Puffed foods | 0.2 | ||||||
4 | Vitamine E | Antioxidant | 06.03.02.04 | Flour pastes (e.g., drag flour pastes used for fish and poultry), breading and frying flour | 0.2 | 1) No acceptance information found; 2) Issued for public comments on October 28, 2022; 3) Officially approved on March 2, 2023 according to No. 1 of 2023. | ||
5 | Ascorbyl palmitate (enzymatic) | Antioxidant | 06.07 | Instant rice flour products | 0.2 | 1) No acceptance information found; 2) Issued for public comments on December 8, 2022. 3) Officially approved on March 2, 2023 according to No. 1 of 2023. | ||
6 | Calcium lactate | Stabilizer coagulant, and acidity regulator | 04.02.02.03 | Pickled vegetables | 10.0 | 1) Accepted on January 5, 2023. Acceptance code: 衛食添新申字(2023)第0002號; 2) Issued for public comments on April 24, 2023. 3) Officially approved on October 7, 2023 according to No. 8 of 2023. | ||
04.02.02.04 | Canned vegetables | 3.0 | ||||||
7 | Sanzan Gum | Thickener, stabilizer and coagulant | 01.01.03 | Modified milk | 0.5 | 1) No acceptance information found; 2) Issued for public comments on April 24, 2023. 3) Officially approved on October 7, 2023 according to No. 8 of 2023. | ||
14.03.03 | Mixed protein drinks | 0.75 | Count as the state of ready-to-drink, increase the usage of solid drinks according to the dilution ratio. | |||||
14.08 | Flavored drinks | 0.5 | ||||||
(6) Approved processing aids with expanded scope and use (5 types)
S.N. | Name | Function | Applicable scope | Approval status (By the end of December 2023) |
|---|---|---|---|---|
1 | Polydimethylsiloxane and emulsion | Defoamer | Processing technology of collagen casing | 1) Acceptance code: 衛食添新申字(2018)第0018號; 2) Issued for public comments on July 1, 2022; 3) Officially approved on March 2, 2023 according to No. 1 of 2023. |
2 | Magnesium stearate | Lubricating agent, releasing agent, and anti-sticking agent | Tablet pressing process of effervescent tablets | 1) Accepted on February 16, 2022. Acceptance code: 衛食添新申字(2022)第0007號; 2) Issued for public comments on July 1, 2022; 3) Officially approved on March 2, 2023 according to No. 1 of 2023. |
3 | Sulfuric acid | Neutralizing and removing soap | Processing technology of oil | 1) Accepted on December 19, 2022. Acceptance code: 食添新申字(2022)第0095號; 2) Issued for public comments on February 10, 2023; 3) Officially approved on August 1, 2023 according to No. 5 of 2023. |
4 | Edible tannin | Clarifying agent | Processing technology of sugar | 1) Accepted on March 6, 2023. Acceptance code: 衛食添新申字(2023)第0029號; 2) Issued for public comments on June 26, 2023; 3) Officially approved on December 1, 2023 according to No. 10 of 2023. |
5 | Ethyl acetate | Extraction solvent | Processing technology of tea extracts | 1) Accepted on November 11, 2022. Acceptance code: 衛食添新申字(2022)第0083號; 2) Issued for public comments on June 26, 2023; 3) Officially approved on December 1, 2023 according to No. 10 of 2023. |
(7) Approved food nutrition enhancers with expanded scope and use (4 types)
S.N. | Name | Category number | Food name/category | Maximum levels | Approval status (By the end of December 2023) |
|---|---|---|---|---|---|
1 | Vitamin B1 | 14.04.2.01 | Drinks for special uses (including sports drinks and nutritional beverages) | 2 mg/kg-5 mg/kg | 1) Accepted on October 31, 2022. Acceptance code: 衛食添新申字(2022)第0076號; 2) Issued for public comments on December 8, 2022; 3) Officially approved on May 6, 2023 according to No. 3 of 2023. |
2 | Vitamin B2 | 14.04.02.01 | Drinks for special uses (including sports drinks and nutritional beverages) | 2 mg/kg-5 mg/kg | 1) Accepted on October 31. 2022. Acceptance code: 衛食添新申字(2022)第0077號; 2) Issued for public comments on December 8, 2022. 3) Officially approved on May 6, 2023 according to No. 3 of 2023. |
3 | Taurine | 14.04.02.01 | Drinks for special uses (including sports drinks and nutritional beverages) | 0.1 g/kg-0.6 g/kg | 1) Accepted on October 31. 2022. Acceptance code: 衛食添新申字(2022)第0075號; 2) Issued for public comments on December 8, 2022. 3) Officially approved on May 6, 2023 according to No. 3 of 2023. |
4 | Ascorbyl palmitate (enzymatic) | As the chemical compound of Vitamin C, its scope of use and maximum levels shall comply with the provisions of GB 14880. | 1) No acceptance information found; 2) Issued for public comments on December 8, 2022. 3) Officially approved on May 6, 2023 according to No. 3 of 2023. | ||
CIRS opinion
It can be observed from the analysis above that new food enzymes still dominate, comprising the largest share at 35.29%, the majority of which are derived from genetically modified sources. However, the percentage of newly approved food additives (52.94%) this year is higher than that of food additives with expanded use (47.05%). Notably, according to Notice No. 8 of 2023, one Lacto-N-neotetraose of microbial source and three 2’-fucosyllactose of microbial sources, two types of highly anticipated HMOs, were successfully approved this year, which is expected to further enrich the infant formula market.
Compared to the first quarter of 2022, the number of substances accepted in the first quarter of this year has significantly increased, more than doubling from the same period last year, yet most of the review outcomes were unsatisfactory. This indicates that the official standards for the review of food additives have become more stringent. In the future, applicants should be well-prepared in terms of product safety and technical necessity in response to the review process. In the case of products that are not approved, companies can better organize and prepare the necessary files, and resubmit the application based on the review results.
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.

