On July 2, 2025, National Health Commission of the People’s Republic of China (NHC) issued an announcement (No. 4 of 2025) for “Three New Foods” with 20 products approved, including 5 new food raw materials, 9 new food additives, and 6 food-related products.

Details are as follows:
New food raw materials
D-allulose/D-psicose
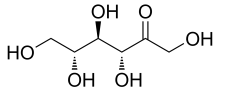
Name | D-allulose/D-psicose |
Basic information | Structure:
CAS No.: 551-68-8 Molecular formua: C6H12O6 Molar mass:180.16 |
Brief introduction of production process | Method One involves using glucose or sucrose as raw materials, which are then fermented, purified, and dried using E. coli AS10. Method Two uses fructose as the raw material, which is catalyzed by the permitted D-psicose 3-epimerase, followed by processes of decolorization, separation, purification, and crystallization. |
Recommended intake | ≤ 20 g/day |
Other information |
|
Saccharomyces cerevisiae CNCM I-3799
Name | Saccharomyces cerevisiae CNCM I-3799 |
Other information |
|
Bifidobacterium animalis subsp.lactis BLa80
Name | Bifidobacterium animalis subsp.lactis BLa80 |
Other information |
|
Bifidobacterium longum subsp. infantis LMG 11588
Name | Bifidobacterium longum subsp. infantis LMG11588 |
Other information |
|
Sodium hyaluronate (extract)
Name | Sodium hyaluronate (extract) | |
Basic information | Chicken comb from Gallus gallus domesticus | |
Brief introduction of production process | The product is manufactured from the combs of Gallus gallus domesticus through a series of processes including chopping, enzymatic hydrolysis, filtration, concentration, purification, drying, and milling. | |
Recommended intake | ≤ 300 mg/day | |
Specifications | Properties | White powder |
Sodium hyaluronate, g/100g | ≥60.0 | |
Chondroitin sulfate, g/100g | ≥5.0 | |
Collagen, g/100g | ≥5.0 | |
Moisture, g/100g | ≤10.0 | |
Ash, g/100g | ≤15.0 | |
PH | 6.0~8.0 | |
Other information | 1.Scope of use: Dairy and dairy products: 0.3 g/kg for formulated milk and flavored fermented milk; for milk powder and its formulated products, the amount is calculated based on the reconstituted liquid mass. Beverages: For liquid beverages packaged in containers of ≤50 mL, up to 3.0 g/kg; for packaging between 51 mL and 500 mL, up to 0.3 g/kg; for solid beverages, the amount is calculated based on the reconstituted liquid mass. Alcoholic beverages: 1.5 g/kg. Cocoa products, chocolate and chocolate products (including compound chocolate and related products) and confectionery: 4.5 g/kg. Frozen desserts: 3.0 g/kg. 2.Not suitable for infants, pregnant women, and lactating women. Labels and instructions must indicate unsuitable populations and consumption limits. 3.Quality specifications and food safety indicators are listed as follow. | |
Pb, mg/kg | ≤0.5 | |
Cd | ≤0.5 | |
Hg | ≤0.05 | |
As, mg/kg | ≤0.3 | |
Cr, mg/kg | ≤5.0 | |
Total colony count | ≤1000 | |
Coliform bacteria | ≤50 | |
Molds and yeasts | ≤150 | |
Salmonella | Not detected | |
Staphylococcus aureus | Not detected | |
New food additives (9 types)
New food enzymes
No. | Enzyme | Source | Donor |
1 | Aminopeptidase | Trichoderma reesei | Aspergillus clavatus |
2 | Xylanase | Trichoderma reesei | Fusariumverticillioides |
3 | Glucoamylase | Aspergillus niger | Trametescingulata |
The quality specifications for enzymes used in the food industry shall comply with the provisions outlined in the National Food Safety Standard for Food Additives – Enzymes for Food Industry (GB 1886.174).
New food additives with expanded scope
No. | Name | Function | Category number | Food name | Maximum level (g/kg) | Note |
1 | Ascorbyl palmitate (enzymatic method) | Antioxidant | 13.01 | Infant formula | 0.05 | Calculated as ascorbic acid in the oil/fat |
13.02 | Infant complementary foods | 0.05 | Calculated as ascorbic acid in the oil/fat |
Processing aids with expanded scope
No. | Name | Function | Application scope |
1 | sulfuric acid | wall breaking | Algal cell wall breaking technology |
2 | deacetylated chitin (chitosan) | clarifying agent | Sugar production process |
3 | ethyl acetate | extraction solvent | Processing method for oil derived from algae (residual solvent/content ≤ 20 mg/kg) |
Food additives with supplementary quality specification
2’-fucosyllactose,2’-FL
Application scope, usage level and specifications: The application scope and specification for 2'-Fucosyllactose shall follow the Notice No. 8 of 2023 issued by the National Health Commission (except for the information on the production strains used for 2'-Fucosyllactose production in Appendix C). The information on the production strains for this new nutrition enhancer is provided in the table below.
Nutrition enhancer | Source | Donor |
2’-fucosyllactose | Bacillus subtilis 168 | Bacillussubtilisa Escherichia colib Helicobacter pylori.c |
Escherichia coli BL21(DE3) | Helicobacter pylori.c Escherichia coli O126c | |
Escherichia coli K- 12 MG1655 | Helicobacter pylori.c |
a donor for mannose-6-phosphate isomerase
b donor for phosphomannomutase, mannose-1-phosphate guanylyltransferase, GDP-mannose dehydratase, GDP-fucosyltransferase, lactose permease, and sugar efflux transporter
c donor for α-1, 2-fucosyltransferase
Food additives subject to changes in quality specification
Quality Specification Requirements of Food Additive Magnesium L-sulfate as Stipulated in the 2016 Announcement No. 8 by the Former National Health and Family Planning Commission:
Name | Index | Test method |
Mg, mg/kg | 7.2-8.3 | GB5413.21 |
Changed into
Name | Index | Test method |
Mg, w% | 7.2-8.3 | GB5413.21 |
New food-related products (6 types)
Food contact additives with expanded use scope/level
Name | CAS number | Range of application | Maximum level |
Ammonium salt of dodecanoic acid | 2437-23-2 | Coatings and paints | 0.6 |
Hexanedioic acid, polymer with2- ethyl-2-(hydroxymethyl)-1,3- propanediol | 28301-90-8 | Coatings and paints | 3 |
3-Aminopropyltriethoxysilane | 919-30-2 | Plastic: Unsaturated Polyester Resin (UP) | 0.01125 |
Bis(2-ethylhexyl) adipate | 103-23-1 | Plastic: Polyvinylidene Fluoride (PVDF) | 35 |
Esters of C12~C18 straight chainfattyacids and C12~C18 straight chainfattyalcohols | — | Plastic | 0.5 |
New food contact resin
Name | CAS number | Range of application | Maximum level |
1,3-Benzenedicarboxylic acid, polymer with 1,4-benzenedicarboxylic acid, 1,4-butanediol, 1,4-cyclohexanedimethanol, 1,3- dihydro- 1,3-dioxo-5-isobenzofurancarboxylicacid and 1,2-ethanediol | 1621282-90-3 | Coatings and paints | 30 |
About CIRS Group
Established in 2012, the Food Business Division of CIRS Group has helped over 1,000 domestic and international food companies achieve one-stop compliance solutions. CIRS offers a full range of regulatory services covering novel food applications, synthetic biology-derived foods, U.S. GRAS notice, EU novel food application, health food registration, and food for special medical purposes (FSMP).
Our food services in China include but not limited to:
- China new food raw materials registration
- China new food additive registration
- China health food (dietary supplement) registration/filing
- China health food testing service
- China new food contact substance registration
- China food for special medical purpose (FSMP) registration
- China infant formula milk powder registration
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.