In order to help enterprises better understand the filing status of health food (dietary supplements) in China, CIRS has gathered statistics on health foods approved in 2025 (as of June 30) and analyzed them from multiple perspectives.
1. The Filing Status of Health Food in China
As of June 30, 2025, according to the information released by the Special Food Information Query Platform of the State Administration for Market Regulation (SAMR), 1963 health foods obtained filing certificates, of which 1949 are domestic health foods and 14 are imported health foods (detailed information is shown in Table 1).
Table 1. Filing Information of Imported Health Food
S.N. | Product Name | Applicant | Filing Number | Country |
|---|---|---|---|---|
1 | NOVAFUN®DHA藻油軟膠囊 | America Willings Pharm Ltd | 食健備J202500000001 | America |
2 | 澳新維特®鋅維生素C片 | AUNEWVITA PHARMACEUTICALS PTY LTD | 食健備J202500000002 | Australia |
3 | 澳新維特®鈣維生素D片 | AUNEWVITA PHARMACEUTICALS PTY LTD | 食健備J202500000003 | Australia |
4 | 澳新維特®B族維生片 | AUNEWVITA PHARMACEUTICALS PTY LTD | 食健備J202500000004 | Australia |
5 | 澳新維特®鈣維生素D維生素K片 | AUNEWVITA PHARMACEUTICALS PTY LTD | 食健備J202500000005 | Australia |
6 | 愛司盟牌維生素D軟膠囊 | ESMOND NATURAL, INC. | 食健備J202500000006 | America |
7 | 愛司盟牌B族維生素片 | ESMOND NATURAL, INC. | 食健備J202500000007 | America |
8 | 愛司盟牌多種維生素礦物質片 | ESMOND NATURAL, INC. | 食健備J202500000008 | America |
9 | 愛司盟牌鐵維生素C片 | ESMOND NATURAL, INC. | 食健備J202500000009 | America |
10 | 愛司盟牌鈣維生素D片 | ESMOND NATURAL, INC. | 食健備J202500000010 | America |
11 | 愛司盟牌維生素C咀嚼片 | ESMOND NATURAL, INC. | 食健備J202500000011 | America |
12 | 伊丹納牌鋅維生素C片 | Vitalis Pharma AS | 食健備J202500000012 | Norway |
13 | 保立明嘉®鋅硒維生素C泡騰片 | MING JIA BIOTECH CO., LTD. | 食健備J202500000013 | Taiwan |
14 | 紐樂怡®多種礦物質維生素D片 | COSMAX NBT, INC. | 食健備J202500000014 | Korea |
For the domestic health food that has been filed, CIRS will conduct statistical analysis from the four perspectives of the filing situation of different regions, different enterprises, different dosage forms and different efficacy ingredients.
2. The Filing Status of Health Food in Different Regions
The filing status of domestic health foods varies significantly across different regions in China, including provinces, directly governed municipalities, and autonomous regions. Shandong province obtained a total of 591 health food filing certificates, securing the top spot in terms of the number of filings. Anhui province and Guangdong province secured the second and third positions with 255 and 208 certificates, respectively.
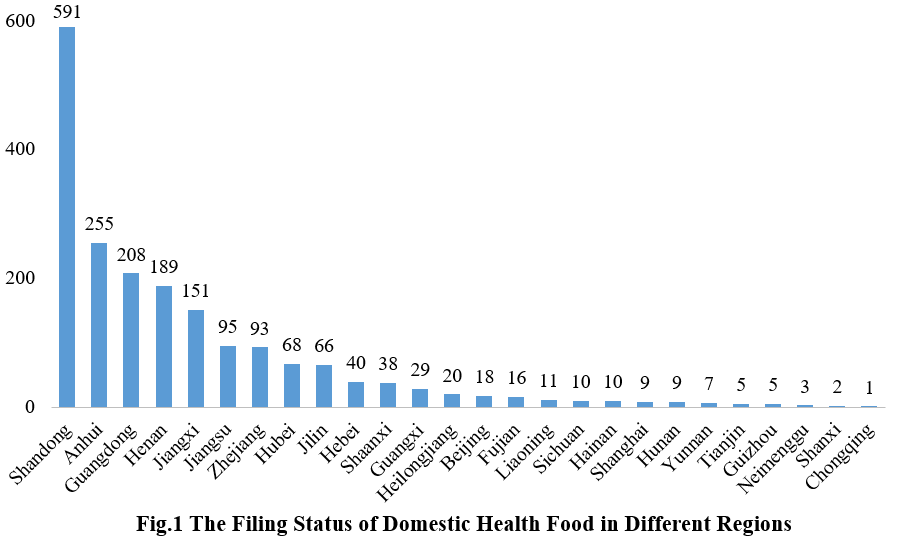
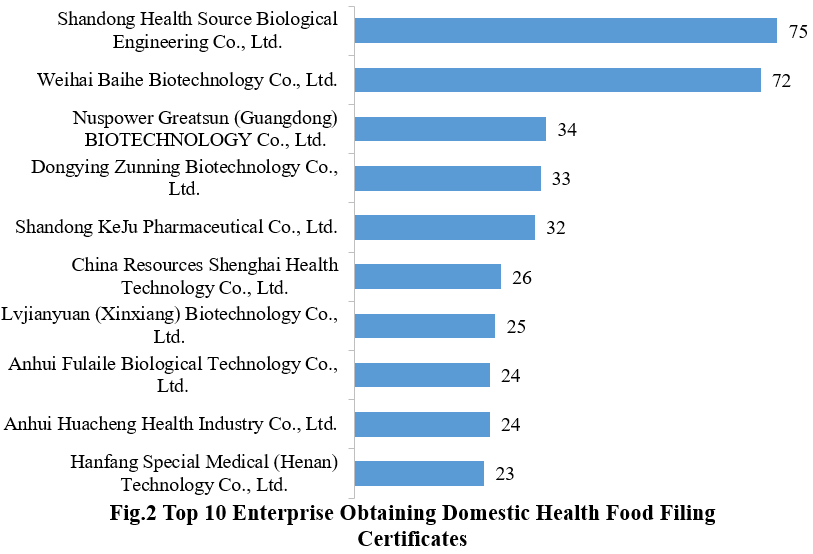
3. The Filing Status of Health Food in Different Enterprises
440 domestic health food enterprises have obtained the filing certificates. Shandong Health Source Biological Engineering Co., Ltd. has the largest number of approvals with a total of 75 filed products, followed by Weihai Baihe Biotechnology Co., Ltd. and Nuspower Greatsun (Guangdong) BIOTECHNOLOGY Co., Ltd., with 72 and 34, respectively.
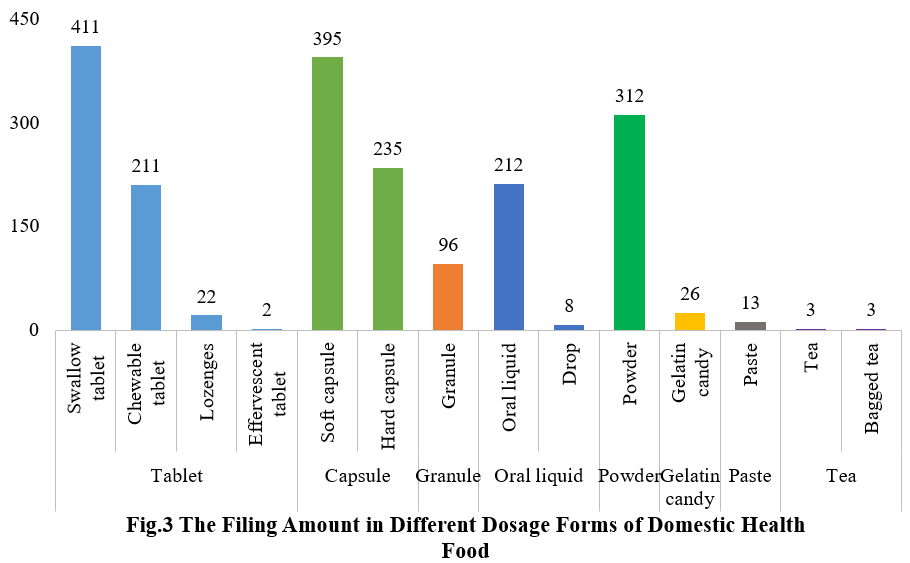
4. The Filing Status of Health Food in Different Dosage Forms
At present, the permitted dosage forms for filing include tablets, capsules (hard/soft), oral liquids, granules, powders, gelatin candy (gummies). Also, when filing products such as panacis quinquefolii radix, ginseng radix et rhizome and ganoderma, mixture, paste and tea (bagged tea) dosage forms can be used.
In the first half of 2025, the main dosage form for domestic health food filing is tablets with 646, accounting for 33.1% of the total. There are 630 filings for capsule products – 395 soft capsules and 235 hard capsules. In addition, the number of oral liquids (including drops) and granule products are 220 and 96 respectively. Among oral liquid products, there are 8 drop products. The quantity of powder and gelatin candy (gummies) products are 312 and 26 respectively. The number of paste and tea products are 13 and 6 respectively. The tea products include 3 bagged tea products.
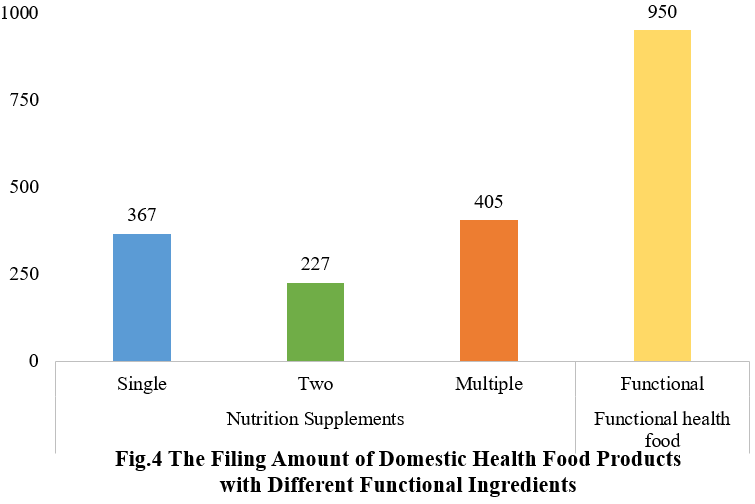
5. The Filing Status of Health Food in Different Functional Components
Among the filed domestic health foods in the first half of 2025, there are 999 nutrition supplements, accounting for 51.3% of the total. The rest are 950 functional health foods with such as broken ganoderma lucidum spore powder as raw material, accounting for 48.7% of the total.
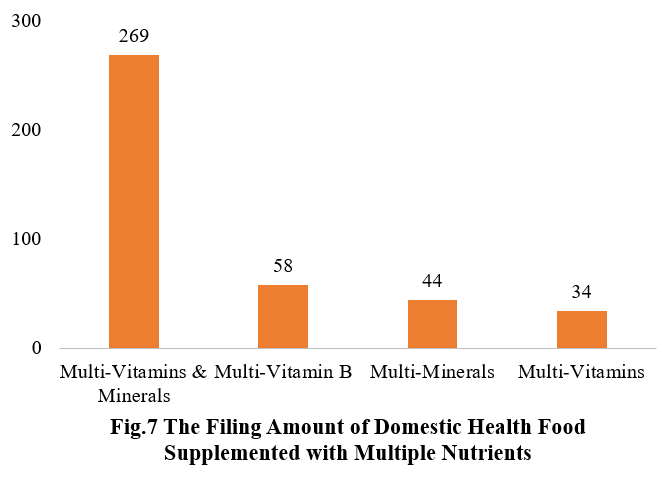
Among the nutrition supplements, products with the function of supplementing multi-vitamins & minerals are greater in quantity (405 products filed), followed by products supplementing a single nutrient (367 products filed) and two nutrients (227 products filed).
Among nutrition supplements, the three major products are selenium supplements, calcium & vitamin D supplements, and multi-vitamins & minerals supplements, which are 83, 81, and 269 respectively.
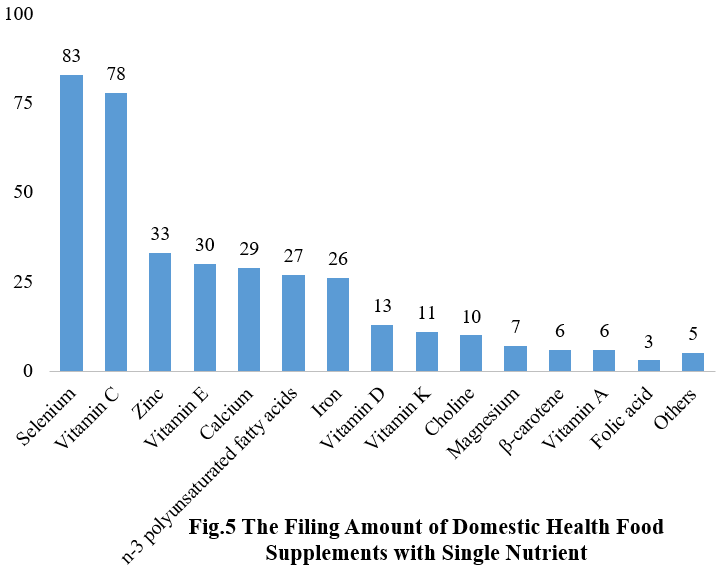
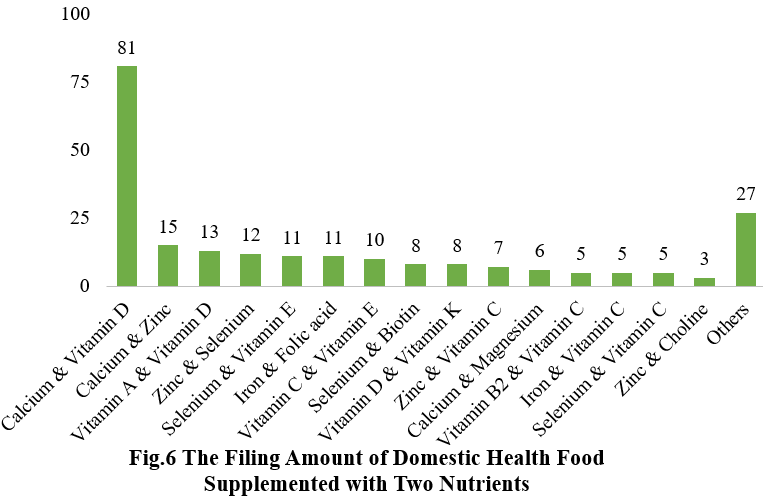
Note: Nutrition supplements with less than three products are classified as “Others”.
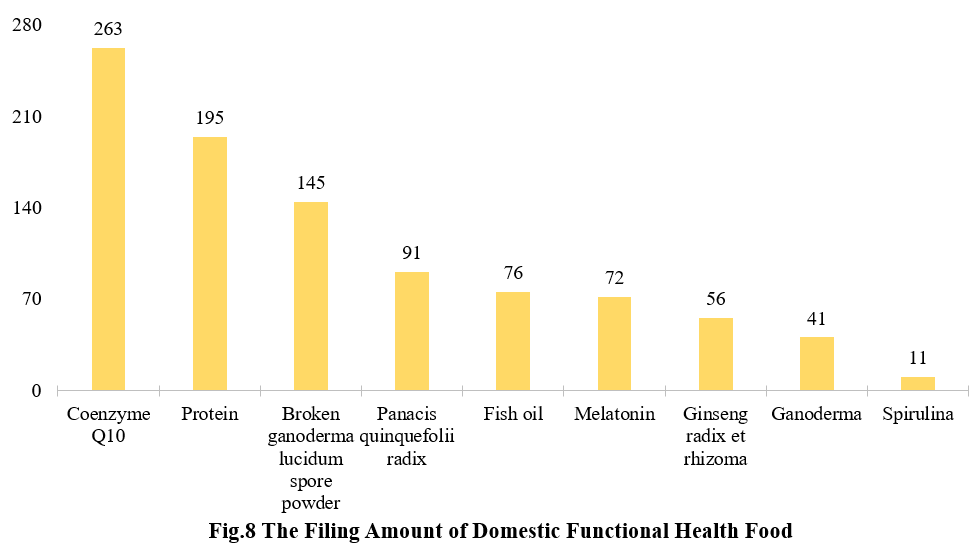
There are 263 filed products with coenzyme Q10 as raw materials have been added. Among the rest functional health foods, products with protein (soy protein isolate and/or whey protein), broken ganoderma lucidum spore powder rank second and third place with 195 and 145 respectively.
CIRS Comments
In the first half of 2025, imported health foods using DHA algal oil as the raw material and effervescent tablets as the dosage form obtained their first batch of filing certificates. Among domestic health foods, the number of functional health products using raw materials such as ginseng radix et rhizoma, panacis quinquefolii radix and ganoderma has increased significantly, also introducing new dosage forms like paste and tea products. In October 2024, a public solicitation of opinions was conducted to propose expanding the dosage forms for health food filings. It is believed that, driven by policy standardization and market demand, filing products will become more scientific and diversified.
Note:
I. The data in this article is from the Special Food Information Query Platform.
II. There may be some omissions in the data of the Special Food Information Query Platform, thus the data in this article is for reference only, and please refer to the information published by the government.
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.

