On August 1, 2025, the European Court of Justice (ECJ) delivered its final judgment in the long-running legal dispute over the safety classification of specific powdered titanium dioxide (TiO?). The court dismissed appeals filed by both France and the European Commission, thereby upholding a 2022 EU General Court ruling that revoked the substance’s classification as carcinogenic. This decision brings a temporary close to years of debate surrounding TiO?’s alleged carcinogenicity.
Chronology of the Controversy
Titanium dioxide, widely used in coatings, pharmaceuticals, and food products for its exceptional opacity and whiteness, became the subject of regulatory scrutiny in 2016. That year, France’s Agency for Food, Environmental and Occupational Health & Safety (ANSES) submitted a proposal to the European Chemicals Agency (ECHA) to classify powdered TiO? as an "inhalation carcinogen." In 2017, ECHA’s Risk Assessment Committee (RAC) endorsed this classification, supporting its categorization as a "Category 2 suspected carcinogen."
In October 2019, the European Commission adopted Delegated Regulation (EU) 2020/217, formally listing "powdered titanium dioxide (containing ≥1% particles ≤10 micrometers)" as a Category 2 carcinogen and mandating the addition of the H351: Suspected of causing cancer when inhaled warning label. The classification and labeling criteria at the time were as follows:
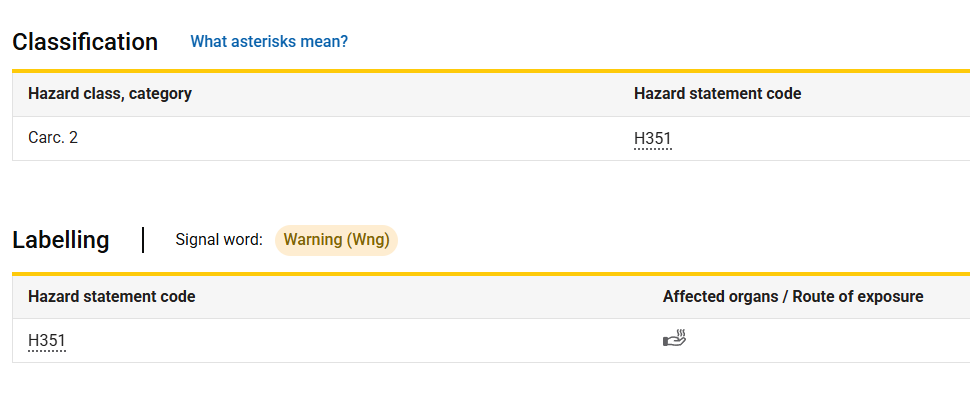
Shortly after the regulation took effect, multiple TiO? manufacturers, importers, and downstream users filed lawsuits with the EU General Court. In November 2022, the General Court ruled that the European Commission had committed "manifest errors" in adopting key scientific studies and failed to sufficiently demonstrate TiO?’s inherent carcinogenic properties. The court thus annulled the classification. France and the European Commission subsequently appealed to the ECJ.
Key Ruling
The ECJ’s August 1 judgment emphasized that the animal study data provided by the European Commission (showing lung tumors in rats exposed to TiO?) could not be directly extrapolated to human carcinogenicity. The court concluded that TiO?’s alleged carcinogenicity stemmed primarily from the particle overload phenomenon (a non-specific mechanism caused by excessive particle accumulation in lungs) rather than intrinsic carcinogenic properties. It ruled that the original classification lacked robust scientific justification.
While the ECJ noted that the General Court had "exceeded the scope of judicial review," it affirmed that annulling the classification and labeling was ultimately justified. The General Court, it stated, was fully entitled to determine that the RAC had not adequately considered all critical factors in evaluating relevant scientific research.
Next Steps
The Commission may reassess the hazards of TiO?, provided it follows rigorous scientific-evaluation procedures. The CJEU stressed that any new classification must rest on “sufficient, reliable and comprehensive scientific data”. At present, no timetable exists at the EU level for an immediate reevaluation.
For companies exporting TiO?-containing products to the EU, the ruling removes the obligation to affix the H351 warning label (“May cause cancer by inhalation”)—a significant boost for the industry.
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.
Further Information

