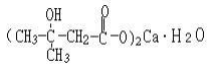In 2022, the National Health Commission of the People's Republic of China (NHC) issued 3 announcements for “Three New Foods” (No.1, 2022; No.2, 2022; No.5, 2022), with 75 products approved (including new food raw materials, new food additives and products related to food). Among the 75 products, five new food raw materials were approved, these are:
- Kanzan flower,
- Pyrroloquinolinequinonedisodium (PQQ) salt,
- Chlamydomonas reinhardtii,
- Bifidobacterium longum subsp.longum BB536, and
- Sugarcane Polyphenols.
In addition, according to the List for the Products on Termination of Review issued by NHC, the approval process for three raw materials were terminated in 2022, these are:
- Penthorum chinense Pursh,
- Sialic acid, and
- Gua Lou Zi.
According to the statistics from the NHC and the China National Center for Food Safety Risk Assessment (CFSA), a total of 22 new food raw materials applications were accepted in 2022, three public consultations on new food raw materials were released. Furthermore, four substances accepted in 2019/2021/2022 were rejected for approval in 2022.
This article will provide you with more information about the acceptance and approval status of new food raw materials in China in 2022.
1. List of new food raw material applications accepted in 2022 (22 types)
In 2022, the NHC accepted 22 applications for new food raw materials, including 16 domestic products and 6 imported products. The technical review status is shown in the following table.
S/N | Acceptance Date | Acceptance Number | Name | The technical review status |
1 | 17/01/2022 | 衛食新申字(2022)第0001號 | Penthorum chinense Pursh | Be included in the termination review list |
2 | 17/03/2022 | 衛食新申字(2022)第0002號 | (3R, 3'S) -dihydroxy-β- carotene | Not yet issue for public comments |
3 | 29/03/2022 | 衛食新進申字(2022)第0001號 | Maquiberry Extract | Not yet issue for public comments |
4 | 24/04/2022 | 衛食新申字(2022)第0003號 | Gua Lou Zi | Be included in the termination review list |
5 | 07/05/2022 | 衛食新申字(2022)第0004號 | Peach gum | Not yet issue for public comments |
6 | 23/05/2022 | 衛食新申字(2022)第0005號 | Pinus massoniana Lamb. | Rejected |
7 | 06/06/2022 | 衛食新申字(2022)第0006號 | Corolla leaf | Not yet issue for public comments |
8 | 09/06/2022 | 衛食新申字(2022)第0007號 | Brown Sugar Oligofructose | Not yet issue for public comments |
9 | 26/07/2022 | 衛食新申字(2022)第0008號 | Yeast protein | Not yet issue for public comments |
10 | 11/08/2022 | 衛食新申字(2022)第0009號 | Periploca sepium Bunge leaves | Rejected |
11 | 12/08/2022 | 衛食新進申字(2022)第0002號 | D-Psicose/D-Allulose | Not yet issue for public comments |
12 | 05/09/2022 | 衛食新申字(2022)第0010號 | Cyperus esculentus var. sativus Boeckeler | Not yet issue for public comments |
13 | 14/09/2022 | 衛食新進申字(2022)第0003號 | Cherry Blossom Extract | Not yet issue for public comments |
14 | 19/09/2022 | 衛食新申字(2022)第0011號 | Bifidobacterium lactis BLa80 | Not yet issue for public comments |
15 | 17/10/2022 | 衛食新申字(2022)第0012號 | D-allulose | Not yet issue for public comments |
16 | 02/11/2022 | 衛食新進申字(2022)第0004號 | Yerba mate | Not yet issue for public comments |
17 | 10/11/2022 | 衛食新進申字(2022)第0005號 | Peanut membrane extract | Not yet issue for public comments |
18 | 21/11/2022 | 衛食新申字(2022)第0013號 | D-allulose | Not yet issue for public comments |
19 | 05/12/2022 | 食新進申字(2022)第0006號 | Pichia kluyveri | Not yet issue for public comments |
20 | 12/12/2022 | 衛食新申字(2022)第0014號 | Not yet issue for public comments | |
21 | 19/12/2022 | 衛食新申字(2022)第0015號 | N-Acetylneuraminic acid | Not yet issue for public comments |
22 | 20/12/2022 | 衛食新申字(2022)第0016號 | γ-Aminobutyric acid | Not yet issue for public comments |
2. List of new food raw materials for public comments released by CFSA in 2022 (three types)
In 2022, a total of three new food raw materials passed the technical review of the expert review committee and solicited public opinions, none of which have been formally approved yet.
(1) Arabinoxylan (Draft announcement):
Name | Arabinoxylan |
Basic information | Structure: arabinoxylan structure (fragment)
CAS number: 9040-27-1 |
Brief introduction of the production process | Prepared through pretreatment, edible alkali extraction, desalkali concentration, neutralization precipitation, washing and purification, drying, and other processes by using sugarcane, wheat bran, corn skin, and other grains as raw materials. |
Recommended intake | ≤15 grams per day (g/day) (with arabinoxylan content of 85 g/100 g, the content exceeding the actual content shall be converted) |
Other information | 1. Not suitable for consumption by infants, pregnant women, and breastfeeding women. This should be indicated on the labels and instructions. 2. Quality specifications and food safety indicators are detailed in the draft announcement. |
Issued date | 14/03/2022 |
(2) Leuconostoc pseudomesenteroides (Draft announcement):
Latin Name | Leuconostoc pseudomesenteroides |
Other information | 1. Scope of use: fermented milk, cheese, fermented milk-containing drinks, and lactic acid bacteria drinks. 2. Approved as edible fungi, the scope of use does not include infant food. 3. Food safety indicators should meet the relevant standards of China. |
Issued date | 29/06/2022 CFSA Collected Public Comments on Leuconostoc pseudomesenteroides |
Acceptance date | 19/04/2021, 衛食新進申字(2021)第0002號 |
(3) Calcium alciumnce damethyl butyrate (CaHMB) (Draft announcement):
Name | Calcium β-hydroxy-β-methyl butyrate (CaHMB) |
Basic information | Constitutional formula: Molecular formula: C10H18O6Ca?H2O Molecular weight: 292 |
Brief introduction of the production process | Prepared through oxidation synthesis, acidification, extraction, neutralization reaction, centrifugation, drying, and other processes by using sodium hypochlorite, diacetonol, hydrochloric acid, ethyl acetate, ethanol, calcium hydroxide as the main raw materials. |
Recommended intake | ≤ 6 g/day |
Other information | 1. Scope of use: beverages, milk, and dairy products, cocoa products, chocolate and chocolate products, confectionery, baked goods, sports nutrition food, and formula food for special medical use. 2. Not suitable for consumption by infants, pregnant women, and breastfeeding women. This should be indicated on the labels and instructions with consumption limits. 3. See the draft announcement for quality specifications and food safety indicators. |
Issued date | 21/10/2022 CFSA Collected Public Comments on Calcium β-hydroxy-β-methyl butyrate (CaHMB) |
Acceptance date | 15/06/2020, 衛食新申字(2020)第0003號 |
3. List of approved new food raw materials in 2022 (five types)
In 2022, the NHC approved a total of five new food raw materials, and the details are as follows:
(1) Kanzan flower
Name | Kanzan flower |
Basic information | Source: The flowers of Cerasus serrulata ”Sekiyama". The flowering period is more than 3/4 of the buds to full bloom. |
Other information | 1. Not suitable for consumption by infants, pregnant women, and breastfeeding women. This should be indicated on the labels and instructions. 2. Food safety indicators shall be implemented in accordance with the provisions of other vegetable products. |
Acceptance date | 25/01/2021, 衛食新申字(2021)第0001號 |
Date of soliciting comment | 17/08/2021 CFSA Collected Public Comments on Kanzan flower and Pyrroloquinolinequinonedisodium (PQQ) salt |
Approved date | 01/03/2022, officially approved by the announcement No.1 of 2022 |
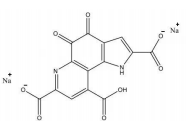
(2) Pyrroloquinolinequinonedisodium (PQQ) salt
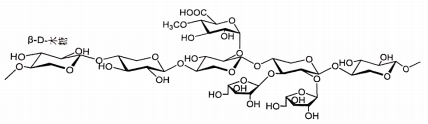
Name | Pyrroloquinolinequinonedisodium (PQQ) salt | |
Basic information | CAS: 122628-50-6 The formula is: C14H4N2Na2O8 Molecular weight: 374.17 Chemical structural formula:
| |
Brief introduction of the production process | Prepared through coupling ring formation reaction, quinoline ring formation, oxidation reaction and ester hydrolysis reaction, refining, and other processes by using 6-amino-5-methoxy-1H-Indo-2-carboxyl ester and 2-oxidized prenenyl dimethyl ester as the main raw materials. | |
Recommended intake | ≤20 mg/day | |
Quality requirement | Shape and properties | Red-brown powder |
Priroroquinone (dry base), g/100 g ≥ | 98.0 | |
Moisture, g/100 g ≤ | 12.0 | |
Other information | 1. Scope and maximum use: beverage (40 mg/kg, solid beverage is converted according to the liquid volume after preparation). 2. Not suitable for consumption by infants, pregnant women, and breastfeeding women. This should be indicated on the labels and instructions. 3. The method for the determination of the content of pyrroloquinoline quinonedi sodium salt and two impurities (3-chloro-4,5-dioxo-4,5-dihydro-1H-pyrrolo[2,3-f]quinoline-2,7,9-tricarboxylic acid and 4-nitro-5-methoxy-1H-pyrrolo[2,3-f]quinoline-2,7,9-tricarboxylic acid) is described in the bulletin. 4. Food safety indicators shall comply with the following provisions: | |
Lead (Pb), mg/kg ≤ | 0.5 | |
Arsenic (As), mg/kg ≤ | 1.0 | |
Cadmium (Cd), mg/kg ≤ | 0.1 | |
Mercury (Hg), mg/kg ≤ | 0.1 | |
3-Chloro-4,5-dioxo-4,5-dihydro-1H-pyrrolo[2,3-f]quinoline-2,7,9-tricarboxylic acid, g/100g ≤ | 0.8 | |
4-Nitro-5-methoxy-1H-pyrrolo[2,3-f]quinoline-2,7,9-tricarboxylic acid, g/100g ≤ | 0.5 | |
Acceptance date | 08/03/2021, 衛食新申字(2021)第0004號 | |
Issued date | 17/08/2021 CFSA Collected Public Comments on Kanzan flower and Pyrroloquinolinequinonedisodium (PQQ) salt | |
Approved date | 01/03/2022, officially approved by the announcement .1 of 2022 | |
(3) Chlamydomonas reinhardtii
Latin Name | Chlamydomonas reinhardtii | |
Basic information | Species: Chlamydomonas family, Chlamydomonas | |
Brief introduction of the production process | Made by algal species cultivation, fermentation tank heterotrophic expansion cultivation, drying, and other processes. | |
Quality requirement | Shape and properties | Green powder |
Protein content | ≥30.0% | |
Crude polysaccharide content | ≥10.0% | |
Other information | 1. The scope of use does not include infant food. 2. Food safety indicators shall be implemented in accordance with the current national food safety standard on algae and algae products. | |
Acceptance date | 13/09/2021, 衛食新申字(2021)第0011號 | |
Issued date | 08/12/2021 | |
Approved date | 11/05/2022, officially approved by the announcement No.2 of 2022 | |
(4) Bifidobacterium longum subsp .longum BB536
Latin Name | Bifidobacterium longum subsp .longum BB536 |
Other information | 1. Approved for use in infant food. 2. Food safety indicators should meet the relevant standards of China. |
Acceptance date | 18/05/2021 , 衛食新進申字(2021)第0003號 |
Issued date | 01/11/2021 CFSA Collected Public Comments on Bifidobacterium longum subsp .longum BB536 |
Approved date | 11/05/2022, officially approved by the announcement No.2 of 2022 |
(5) Sugarcane polyphenols
Name | Sugarcane Polyphenols |
Brief introduction of the production process | Prepared by using sugarcane as raw material through crushing, filtration, extraction, removing excess sugar and salt, using water and ethanol as an extraction solvent to be transferred into powder by filtration, vacuum concentration, ion exchange, spray drying, and other processes; or to be transferred into liquid by filtration, vacuum distillation, and other processes. |
Recommended intake | ≤ 1 g/day (powder); ≤ 10 g/day (liquid) The recommended amount of powder (polyphenol content: 200 g/kg) is 1 g/day, and that of liquid (polyphenol content: 14.8 g/kg) is 10 g/day. When the polyphenol content exceeds the above, it shall be converted according to the actual content. |
Other information | 1. Not suitable for consumption by infants, pregnant women, and breastfeeding women. This should be indicated on the labels and instructions. 2. Quality specifications and food safety indicators are detailed in the announcement. |
Acceptance date | 18/05/2021, 衛食新申字(2021)第0008號, Name of acceptance: Sugarcane Extract |
Issued date | 01/11/2021 CFSA Collected Public Comments on Bifidobacterium longum subsp .longum BB536 |
Approved date | 11/05/2022 officially approved, the announcement is No.2 of 2022 |
4. List of terminated reviewed products in 2022 (3 types)
In 2022, a total of three new food raw materials were included in the termination review list which contains 68 products at present. For the enterprises applying for new food raw materials, getting the review results of "ordinary food", "substantial equivalent" and "local traditional eating habits" is also a way to "get approval". The relevant termination review opinions are as follows.
S/N | Product Name | Review Opinions | Acceptance Date and Number |
1 | Penthorum chinense Pursh | The NHC has approved penthorum chinense pursh as a new food raw material for bubble drinking in Announcement No.4 of 2020, and the application scope is applying for expanding to the beverage. Based on the safety assessment data provided, it was agreed to use the beverage and recommended to terminate the review. Except for the edible form, it shall be implemented in accordance with No.4 of 2020. | 17/01/2022, 衛食新申字(2022)第0001號 |
2 | Gua Lou Zi | Gua Lou Zi has been included in the "National Food Safety Standard Nuts and Seed Food" GB 19300, used as food raw materials according to the management of nuts and seed food, it was recommended to terminate the review. | 24/04/2022, 衛食新申字(2022)第0003號 |
3 | Sialic acid | Prepared through bacterial fermentation, filtration, sterilization, hydrolysis, ultrafiltration, decolorization, decontamination, nanofiltration, concentration, purification, drying, crushing, sieving, and other processes by Escherichia coli (strain No. CASOV-09) as the fermenting strain. The production process is essentially the same as that of Sialic acid approved for the announcement (formerly Health and Welfare Commission Announcement No. 7 of 2017), which shows substantial equivalence. Other requirements are implemented in accordance with the relevant content of the published announcement of Sialic Acid, and food safety indicators are implemented in accordance with the following: Aflatoxin B1≤5 micrograms per kilogram (μg/kg), Lead (calculated as Pb) ≤0.8 milligrams per kilogram (mg/kg), Mercury (calculated as Hg)≤0.2 mg/kg, Arsenic (calculated as As)≤0.4 mg/kg, total colony count ≤1000 CFU/g, Coliform ≤0.6 MPN/g, Mould≤100 CFU/g, Yeast≤100 CFU/g, Salmonella 0/25 g, Staphylococcus aureus 0/25 g. | 06/04/2021, 衛食新申字(2021)第0007號 |
5. List of new food raw materials not approved and issued an administrative license by NHC in 2022 (three types)
According to the publicly available information of NHC, there are four new food raw materials not approved and issued an administrative license in 2022, including one product that submitted its application in 2019, two products in 2021, and one product in 2022. The details of each product are shown in the following table.
S/N | Product Name | Acceptance Date | Acceptance Number | Review Process / Conclusion |
1 | Mycelium Oxalicum Powder | The acceptance date was not reached | 衛食新進申字(2019)第0002號 | 18/02/2022 Issuance of a decision not to grant administrative permission |
2 | Isomaltodextrin | 12/04/2021 | 衛食新進申字(2021)第0001號 | 01/06/2021 Delivering a notification of review opinion on the new food raw material 22/04/2022 Issuance of a decision not to grant administrative permission |
3 | Maize ferments | 19/07/2021 | 衛食新進申字(2021)第0004號 | 30/07/2021 Delivering a notice on delaying review for the new food raw material 08/10/2021 Delivering a notice on delaying review for the new food raw material 02/12/2021 Delivering a notice on delaying review for the new food raw material 17/02/2022 Delivering a notice on delaying review for the new food raw material 26/04/2022 Issuance of a decision not to grant administrative permission |
4 | Pine (Pinus massoniana Lamb.) needle | 23/05/2022 | 衛食新申字(2022)第0005號 | 27/06/2022 Delivering a notification of review opinion on the new food raw material 26/10/2022 Issuance of a decision not to grant administrative permission |
Summary
Seeing that the Pyrroloquinolinequinonedisodium (PQQ) salt and Sugarcane Polyphenols were approved within one year and kept arousing discussions, the new food raw materials with certain functions or benefits are showing their superiority in the declaration of compliance and the food industry. For more details, please refer to the article New food raw materials are providing new vitality for the functional food industry by CIRS.
On August 25, 2022, the NHC issued an announcement on the update of List of Strains that Can be Used in Food and List of Strains that Can be Used for Infants and Children (No.4, 2022). The classification and nomenclature of the strains involved in the two lists will be adjusted and a two-year transitional period will be set. According to the official interpretation, both the old and new strain names can be used during the transitional period, after which only the updated lists of strains can be used.
As one of the "three new foods", new food raw materials (novel food) have the most complex and stringent requirements in declaration and review. Enterprises planning to carry out an application should set out early with adequate preparations of dossiers.
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.
(Note: There may be omissions and errors in the data statistics process because of the change of the official website. The data in this article is for reference only. Please refer to the official information published by the government.)