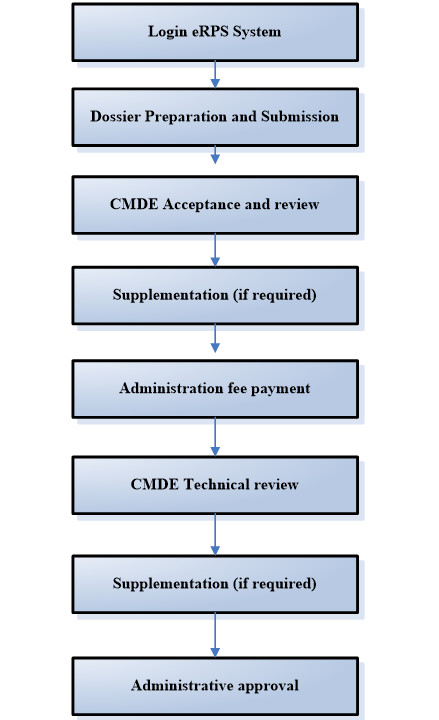
China National Medical Product Administration (Known as NMPA) has organized the development of the Electronic Regulated Product Submission (eRPS) system and officially launched on June 24 2019, all registration should be submitted through eRPS system from November 1 2019.
Scope of application
- Medical device initial registration
- Change in registration of class II or III medical devices
- Registration renewal of class II or III medical devices
- Approval of clinical trials for Class III high-risk medical devices
- Notification for revising medical device instructions
- Re-review of registration of medical devices
- Change of licensing items
- Special review of innovative medical devices
The record filing of imported Class I medical devices; the replacement, correction, self-deregistration, and self- revocation of registration certificates and change documents; and the designated test for medical device registration, etc., are not included in the scope of the eRPS system.
Time Schedule on eRPS implementation
Time Schedule | System startup |
May 2019 | the applicants and registrants of medical device registration can apply for a Certificate Authority (CA) for use in the eRPS system |
June 24 2019 | the eRPS system was officially launched, Medical device registration applicants and registrants can apply for online electronic registration of medical devices without submitting paper dossiers. Meanwhile, NMPA reserves the means of submitting paper dossiers. |
Prior to October 31 2019 | The submission of paper materials may follow the current requirements for registration of medical devices and in vitro diagnostic reagents |
From November 1 2019 | The submission of paper materials shall abide by the requirements of the Technical Guidelines for Electronic Submission of Medical Device Registration Applications (Interim) in alignment with the electronic filing form |
Procedure of Submission through eRPS System