Updated on 23 December, 2025
At present, many companies are applying for cannabidiol (CBD) as a novel food in the EU. On 3 November 2025, EFSA released a safety assessment on one synthetic CBD. According to the assessment conducted by EFSA’s expert panel, the safety of this NF synthetic CBD cannot be established due to insufficient data.

Figure 1. Safety assessment published on the EFSA journal website
Reasons for non-approval of this NF synthetic CBD
(1) Lack of additional data from the applicant
During the evaluation, the panel identified multiple data gaps (including information on nanoparticle characteristics, specifications and analytical laboratory accreditation, stability, genotoxicity, reproductive and developmental toxicity, and human data). Recently, EFSA contacted the applicant three times—on 24 March 2025, 7 April 2025, and 24 April 2025—but received no response.
(2) Safety Cannot be established
After reviewing all available evidence, the EFSA panel concluded that the safety of this synthetic CBD as a novel food cannot be established.
 Figure 2. EFSA conclusions of the above-mentioned CBD
Figure 2. EFSA conclusions of the above-mentioned CBD
Current regulatory status of CBD
According to EU Regulation 2015/2283, CBD is classified as a novel food. To be legally placed on the market for use in foods and food supplements within the EU, CBD must obtain formal authorization from the European Union. Based on publicly available information from Open EFSA, more than 60 applications related to CBD as a novel food have been submitted to date. Many applications have failed due to the applicants’ failure to provide sufficient data. However, some companies continue to actively pursue novel food applications, which are currently undergoing full scientific assessment by EFSA (as shown in Figure 3; for example, application EFSA-Q-2020-00257 is currently in the “ongoing risk assessment” stage).
In summary, based on past experience, providing EFSA with comprehensive and complete toxicological data, together with supporting safety evidence is essential for the smooth progression of the scientific assessment. Therefore, companies must ensure their safety assessments and dossier preparation are comprehensive and sufficient.
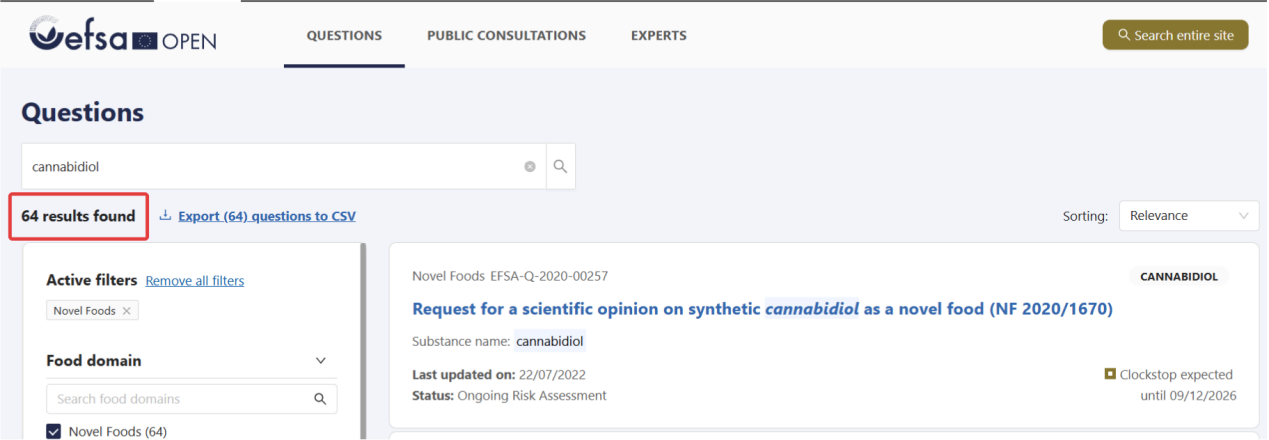 Figure 3. Status of NF CBD applications in the EU
Figure 3. Status of NF CBD applications in the EU
About CIRS Group Food Team
Established in 2012, the Food Business Division of CIRS Group has helped over 1,000 food companies globally achieve one-stop compliance solutions. CIRS offers a full range of regulatory services covering novel food applications, synthetic biology-derived foods, U.S. GRAS notice, EU novel food application, health food registration, and food for special medical purposes (FSMP).
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.

