
On June 30, 2025, the Scientific Committee on Consumer Safety (SCCS) of the European Union issued scientific opinion (SCCS/1671/24) on Ethylhexyl Methoxycinnamate (EHMC) (CAS No. 5466-77-3/83834-59-7, EC No. 226-775-7/629-661-9).
Scientific Advice on the Safety of Ethylhexyl Methoxycinnamate (EHMC)
The SCCS concludes the following:
The SCCS has noted that the available evidence shows that EHMC is an endocrine-active substance due to estrogenic activity and weak anti-androgenic activity both in vitro and in vivo. Having considered the data provided, and the concerns relating to potential endocrine disrupting properties of EHMC, the SCCS is of the opinion that EHMC is safe when used as a UV filter up to a maximum concentration of 10% in sunscreen lotion, face and hand cream, lipstick, sunscreen propellant spray and pump spray, when used separately or in combination.
The SCCS is of the opinion that these products are also safe for children due to the high Margin of Safety, which precludes any difference between internal exposures in children that might be higher due to a different surface/body weight ratio than in adults.
The SCCS mandate does not address environmental aspects. Therefore, this assessment did not cover the safety of EHMC for the environment.
Regulatory Requirements Interpretation of Ethylhexyl Methoxycinnamate (EHMC)
The Global Cosmetic Ingredient Regulatory Database – Global CosIng – independently developed by the CIRS Group, indicates that Ethylhexyl Methoxycinnamate (EHMC) (CAS No. 5466-77-3/83834-59-7) is included in the following key lists globally.
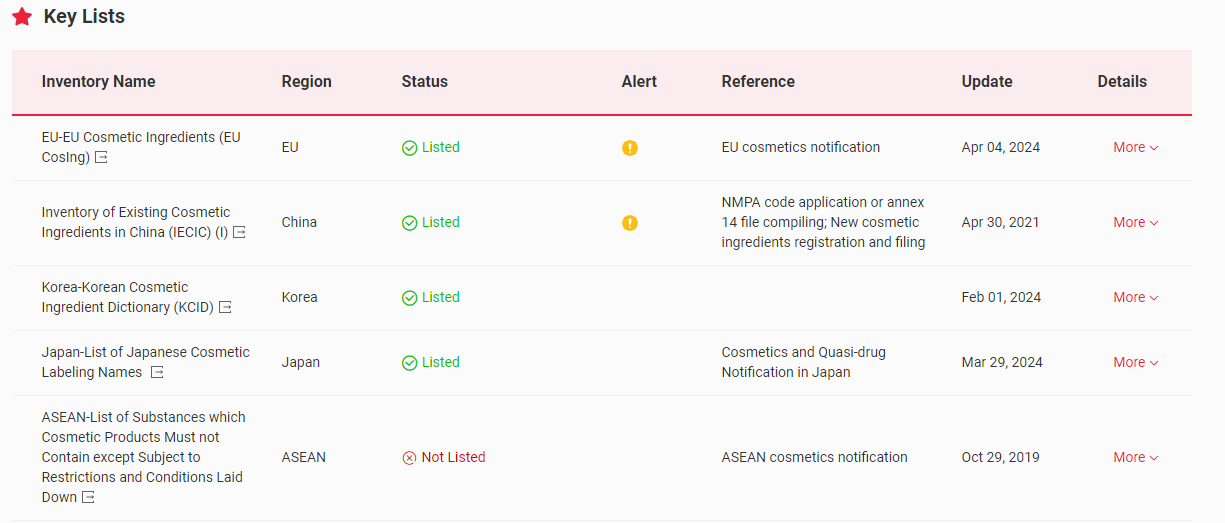
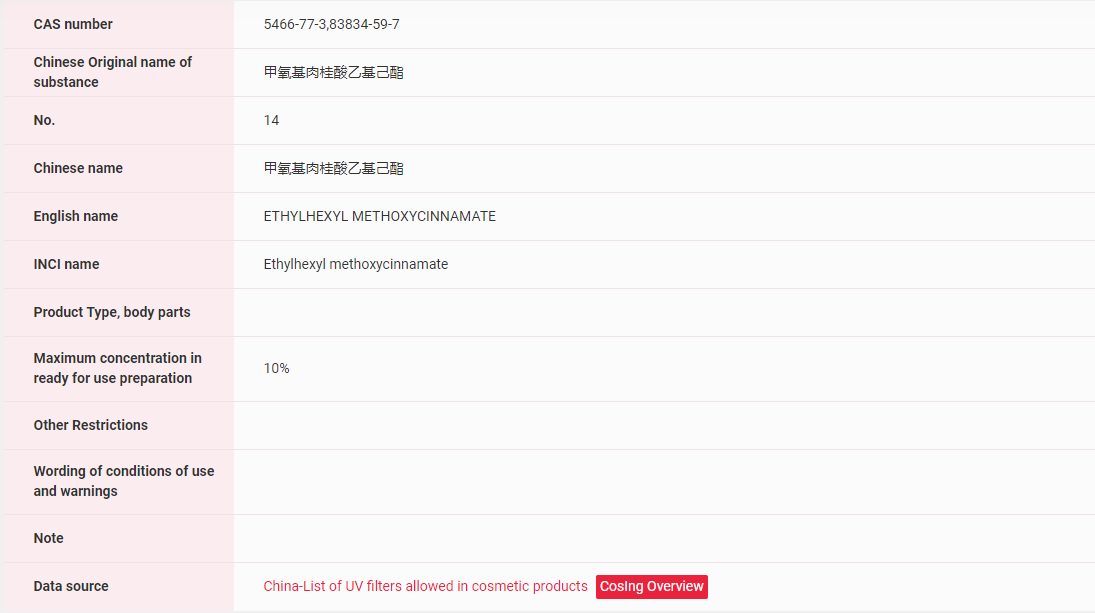
【EU】In the EU, EHMC has been included in the List of UV filters allowed in cosmetic products, the maximum concentration in ready for use preparation is 10%.
China】In China, EHMC has been included in the Inventory of Existing Cosmetic Ingredients in China (IECIC) (I) and List of hair-dyes allowed in cosmetic products, the maximum concentration in ready for use preparation is also 10%.
About CIRS
The CIRS cosmetic team is dedicated to ensuring that cosmetic products meet stringent global regulatory standards. It can provide one-stop services covering the whole life-cycle of a personal care product, which includes cosmetic ingredient development, physical/chemical tests, toxicological tests (in vivo & in vitro), efficacy studies (in vivo & in vitro), ingredient registration, and product registration.
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.
Further Information

