
On 19 November 2025, the Scientific Committee on Consumer Safety (SCCS) of the European Union issued preliminary opinion (SCCS/1686/25) open for comments on the safety of Thiomersal (CAS No 54-64-8) and Phenylmercuric salts (CAS No 62-38-4, 94-43-9) as preservatives in cosmetic products. The deadline for comments is set for 21 January, 2026.
Preliminary Opinion on Thiomersal and Phenylmercuric Salts
Considering that the MoS based on renal toxicity as the most sensitive endpoint is below 100, and given that genotoxicity evidence is unclear, these mercury compounds are not considered safe at concentration levels currently permitted in cosmetic products.
Compared with the mercury compounds mentioned before, the potential risk for human health is aggravated by additional exposure to mercuric compounds from sources other than cosmetics.
Interpretation of Thiomersal and Phenylmercuric Salts
The Global Cosmetic Ingredient Regulatory Database-Global CosIng, independently developed by CIRS Group indicates that in EU, Thiomersal and Phenylmercuric Salts have been included in the List of preservatives allowed in cosmetic products. Detailed information could be found in the following figures.

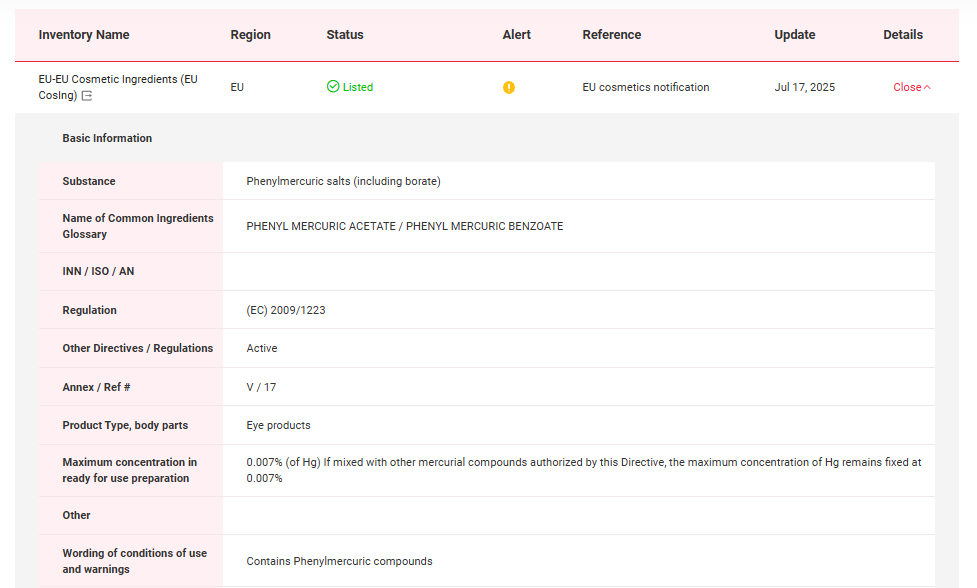
In China, Thiomersal and Phenylmercuric Salts have been included in Inventory of Existing Cosmetic Ingredients in China (IECIC) (I) and List of preservatives allowed in cosmetic products. Detailed information could be found in the following figures.
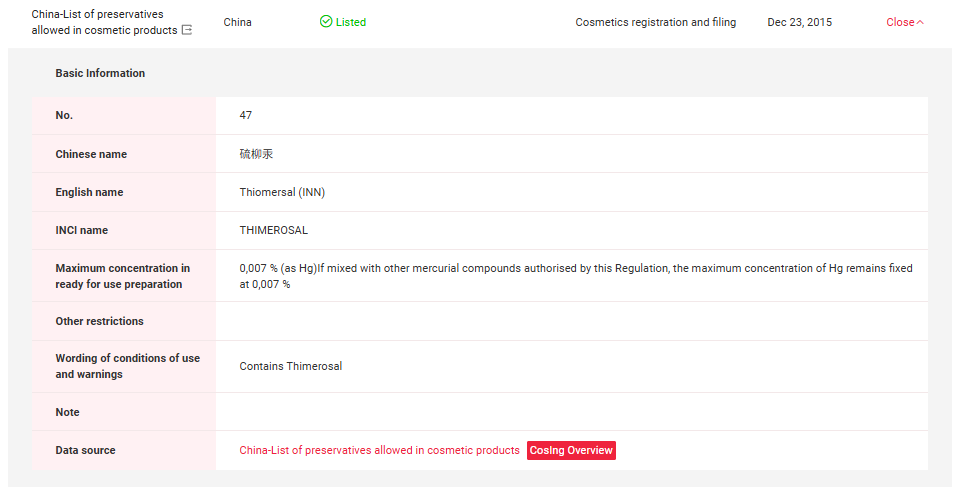
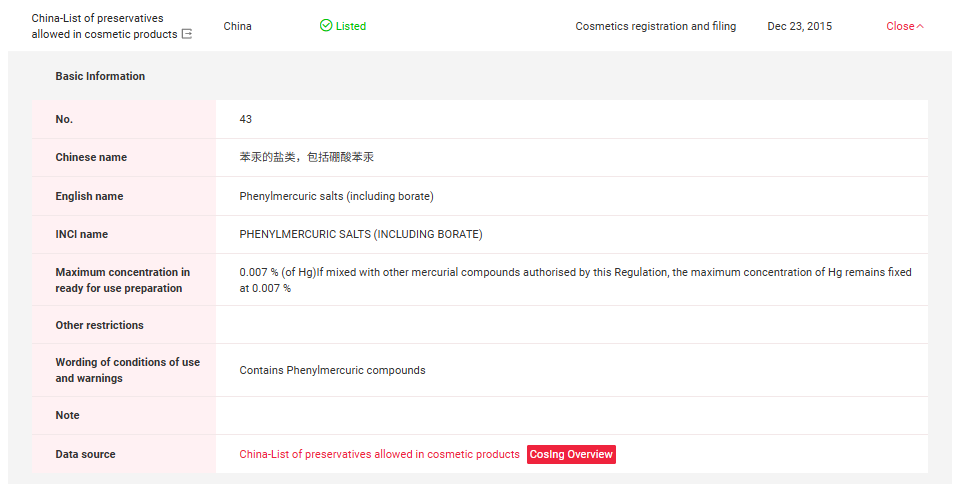
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.
Further Information

