In May 2025, the Scientific Advisory Group of Chemical Safety of Non-food and Non-medicinal Consumer Products (SAG-GS) issued an opinion on 4-Methylbenzylidene Camphor (4-MBC) as a UV Filter in Cosmetic Products.
SAG-GS concludes the following:
Members did not have access to the full industry data package for 4-MBC, even though this had been requested by the OPSS. In the absence of data from industry and an incomplete data package, it was necessary to draw conclusions from the published opinions from other authoritative bodies, such as the SCCS. Members noted that the SCCS was unable to derive a safe level for 4-MBC in cosmetics.
The Members agreed that 4-MBC exhibits endocrine-disrupting properties in in vivo studies. The Members noted concerns with regard to the safety of 4-MBC, including the lack of a robust genotoxicity and reproductive/developmental toxicity package and concerns with regard to endocrine disruption properties. The Members were not able to derive a safe level for the use of 4-MBC in cosmetics.
Given the limited data package available for review and potential concerns for genotoxicity and endocrine disruption, the SAG-CS could not conclude that 4-MBC is safe for use in sunscreen products and other cosmetic products.
Regulatory Requirements Interpretation of 4-MBC
The Global Cosmetic Ingredient Regulatory Database – Global CosIng, an upgraded version of China CosIng independently developed by CIRS Group, indicates that 4-Methylbenzylidene Camphor (CAS No. 38102-62-4/36861-47-9) has been included in the following key lists globally.
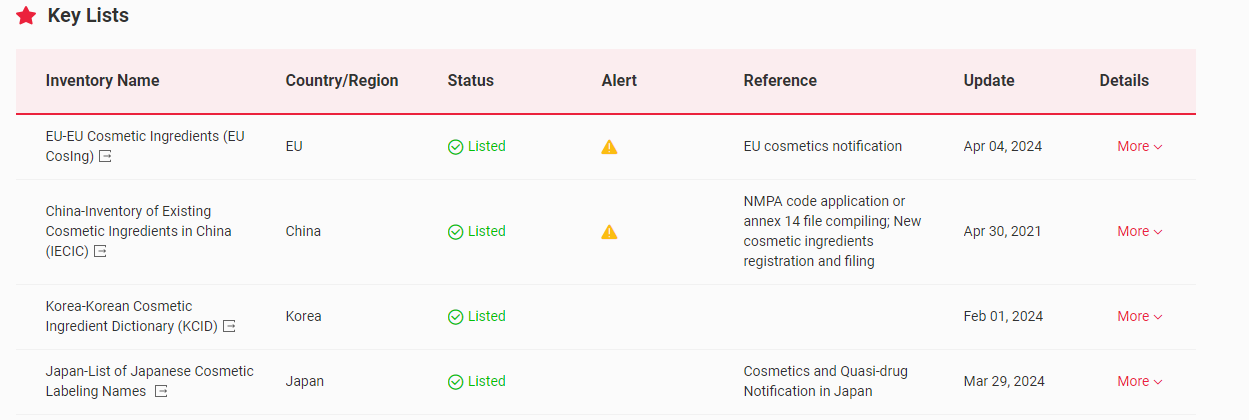
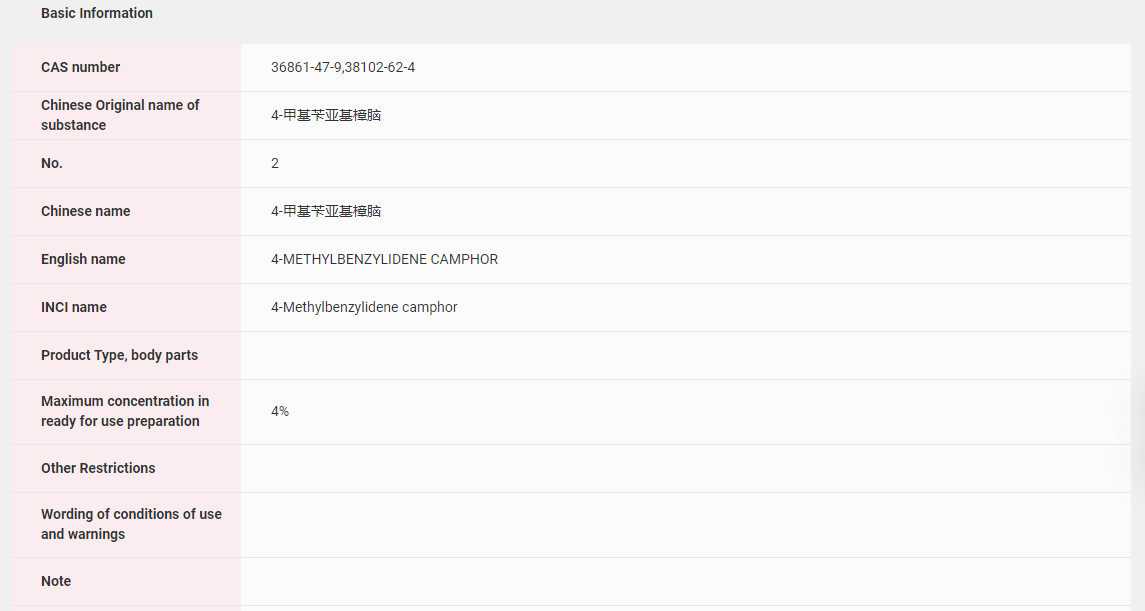
In China, 4-MBC has been included in the Inventory of Existing Cosmetic Ingredients in China (IECIC) and the List of ingredients restricted in cosmetic products, as well as the List of UV filters allowed in cosmetic products in the Safety and Technical Standards for Cosmetics. The maximum concentration in ready-to-use preparations is 4%.
Furthermore, in the UK, 4-MBC has been included on the list of substances permitted for use up to a concentration of 4% as a UV filter within Annex VI (Entry 18) of the Cosmetic Products Regulation UK No 1223/2009 (as amended).
About CIRS
The CIRS cosmetic team is dedicated to ensuring that cosmetic products meet stringent global regulatory standards. It can provide one-stop services covering the whole life-cycle of a personal care product, which includes cosmetic ingredient development, physical/chemical tests, toxicological tests (in vivo & in vitro), efficacy studies (in vivo & in vitro), ingredient registration, and product registration.
Our services
- Responsible Person (RP);
- Preliminary review of formula and label;
- Safety assessment;
- Create an accurate PIF;
- Labelling design; and
- SCPN submission.
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.
Further Information
This article was updated on June 24, 2025.

