In May, 2025, Scientific Advisory Group of Chemical Safety of Non-food and Non-medicinal Consumer Products (SAG-CS) issued opinion on formaldehyde releasing substances (Opinion 16).

SAG-CS concludes the following:
Members concluded that the current threshold of 0.05% for labelling formaldehyde releasing preservatives was insufficient to protect consumers.
The scientific database on this topic is limited, but Members agreed that the data indicate that the current labelling threshold is not protective for the whole population. Members agreed that a threshold of labelling of formaldehyde releasing preservatives of 10 mg/kg (10 ppm) or 0.001% would be appropriate to protectconsumers based on current scientific evidence when using leave-on or rinse-off products.
Regulatory Requirements Interpretation of Formaldehyde
The Global Cosmetic Ingredient Regulatory Database-Global CosIng, an upgraded version of China CosIng independently developed by CIRS Group indicates that Formaldehyde (CAS No.118-56-9) has been included in the following key lists globally.

【UK】Formaldehyde has a harmonised classification as a Category 1B carcinogen under the GB Classification, Labelling and Packaging (CLP) regulation No 1272/2008 (as amended). Furthermore, Formaldehyde is currently included on the list of substances prohibited for use in cosmetic products within Annex II (Entry 1577) of the Cosmetic Products Regulation UK No 1223/2009 (as amended).
Despite the removal of formaldehyde from Annexes III and V of the Cosmetic Regulation, formaldehyde may still be present in cosmetic products through the inclusion of formaldehyde releasers permitted within Annex V. Examples of formaldehyde releasing substances include: DMDM hydantoin (Annex V, Entry 33), imidazolidinyl urea (Annex V, Entry 27), diazolidinyl urea (Annex V, Entry 46), etc. As such, Point 2 within the preamble to Annex V contains the statement: “All finished products containing substances in this Annex and which release formaldehyde must be labelled with the warning ‘contains formaldehyde’ where the concentration of formaldehyde in the finished product exceeds 0.05%”.
【China】Formaldehyde has been included in the Inventory of Existing Cosmetic Ingredients in China (IECIC) and suspected to be listed in the List of ingredients prohibited in cosmetic products. More details could be found in the following figure.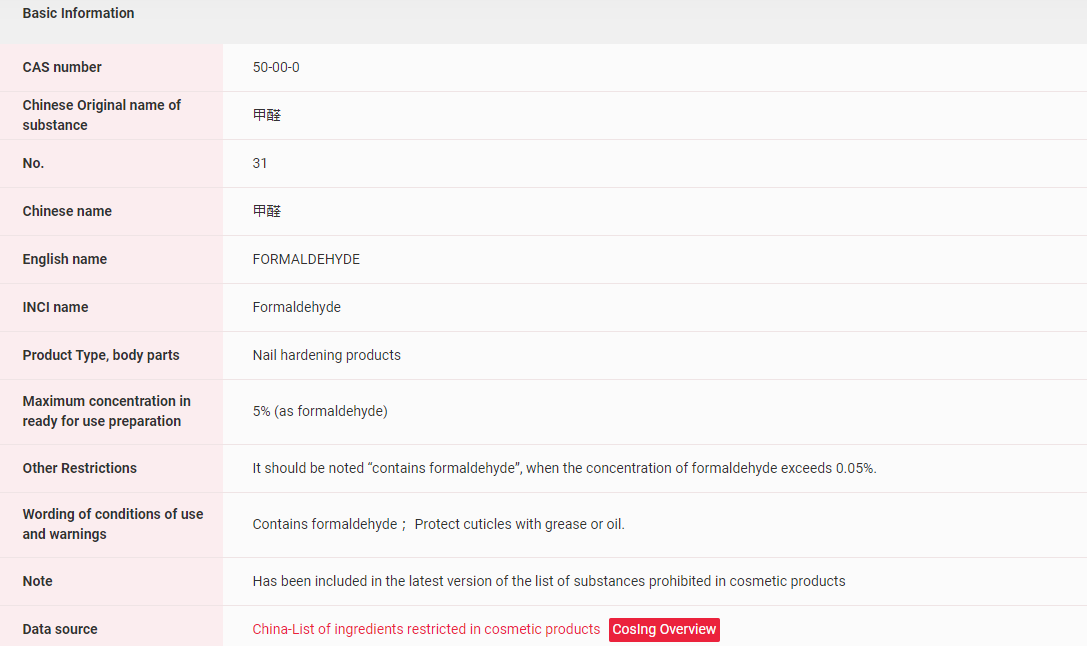
About CIRS
The CIRS cosmetic team is dedicated to ensuring that cosmetic products meet stringent global regulatory standards. It can provide one-stop services covering the whole life-cycle of a personal care product, which includes cosmetic ingredient development, physical/chemical tests, toxicological tests (in vivo & in vitro), efficacy studies (in vivo & in vitro), ingredient registration, and product registration.
Our services
- Responsible Person (RP);
- Preliminary review of formula and label;
- Safety assessment;
- Create an accurate PIF;
- Labelling design; and
- SCPN submission.
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.
Further Information

