Background of K-BPR
The Consumer Chemical Products and Biocides Safety Act is a new chemical regulation proposed by the Ministry of Environment (MoE) in South Korea. Also known as K-BPR, the Act regulates consumer chemical products, biocidal product and biocide-treated articles. It is promuglated on 20 March 2018 and came into force on 1 Jan 2019.
The Consumer Chemical Products and Biocides Safety Act (K-BPR) can be broken into 2 parts: consumer chemical products and biocides. The consumer chemical product part is transferred from K-REACH while the biocide part is taken from EU biocidal products regulation (BPR).
Consumer Chemical Products
Definition
Consumer Chemical Products: Chemical product that is used in our daily life (eg. Home, office,multi-use facility) and may potentially expose people and/or the environment to chemical substance.
Consumer Chemical Product Subject to Safety Check: Consumer chemical product that is designated and announced by the Minister of Environment in recognition of its risks found from risk assessment.
Consumer Chemical Product Subject to Safety Check | |
|---|---|
Cleaning products | Cleaning agent |
Removal | |
Detergent | Laundry detergent |
Bleach | |
Fabric softener | |
Detergent | Glossy coating agent |
Coating agent with specific purpose | |
Anti-rust agent | |
Ironing aid | |
Adhesive | Adhesive |
Sealant | |
Air freshener | Air freshener |
Deodorant | |
Dyes | Material dyes |
Material ink | |
Car maintenance products | Car washer fluid |
Antifreeze | |
Printing products | Printing ink? toner |
Red stamping ink | |
Correction Tape or Correction fluid | |
Beauty products | Beauty adhesive |
Tattoo dye | |
Disinfectants | Disinfectants |
Algaecide | |
Humidifier disinfectants | |
Sterilizer and disinfectant for infectious diseases | |
Other preventive disinfectants | |
Insecticide | Insect repellent |
Prevention, attracting insecticide for hygiene | |
Repellent for hygiene | |
Prevention insecticide for infectious diseases | |
Rodenticide for infectious diseases | |
Preservatives | Wood preservatives |
Preservatives treated filter | |
Others | Candle |
Dehumidifier | |
Artificial snow spray | |
Fog fluid | |
Consumer chemical products for humidifier | |
Main Requirements for Consumer Chemical Products
Anyone who intends to manufacture or import products listed in the table of “Consumer chemical products subject to safety check” shall obtain safety confirmation from the designated test /inspection institutions at interval of every 3 years. After getting confirmation from institutes, they have to submit confirmation application to Korea Environmental Industry & Technology Institute.
Also, any person who intends to manufacture or import Consumer chemical products subject to safety check, of which the safety standards are not announced, shall submit the information of the chemical substance contained in the product to obtain the approval of the MoE such as below product types:
Humidifier disinfectants
Sterilizer and disinfectant for infectious diseases
Other preventive disinfectants
Insect repellent
Prevention, attracting insecticide for hygiene
Repellent for hygiene
Prevention insecticide for infectious diseases
In addition, anyone who intends to sell or distribute Consumer chemical products subject to safety check, of which the safety is confirmed or approved on the market, shall comply with the labeling standards in labeling on the surface of the product.
Biocides (Active substance, Biocidal product, Treated article)
Definition
Active substance (AS): A chemical or natural substance, or micro-organism used for destroying,rendering harmless or dettering the action of any harmful organism
Biocidal product (BP): - product that its primary function is to destroy harmful organisms, etc.
① product containing at least one AS, or product that AS and non-AS (e.g.chemicals, natural substance, micro-organism) are mixed
② product creating biocidal substances by mixing chemical substance,natural substance or micro-organism
Treated article: Product that uses a biocidal product for other function, rather than its primary function.
Main Requirements for Biocides
Manufacturer/importer of active substance and biocidal product shall get approved by the National Institute of Environmental Research (NIER).
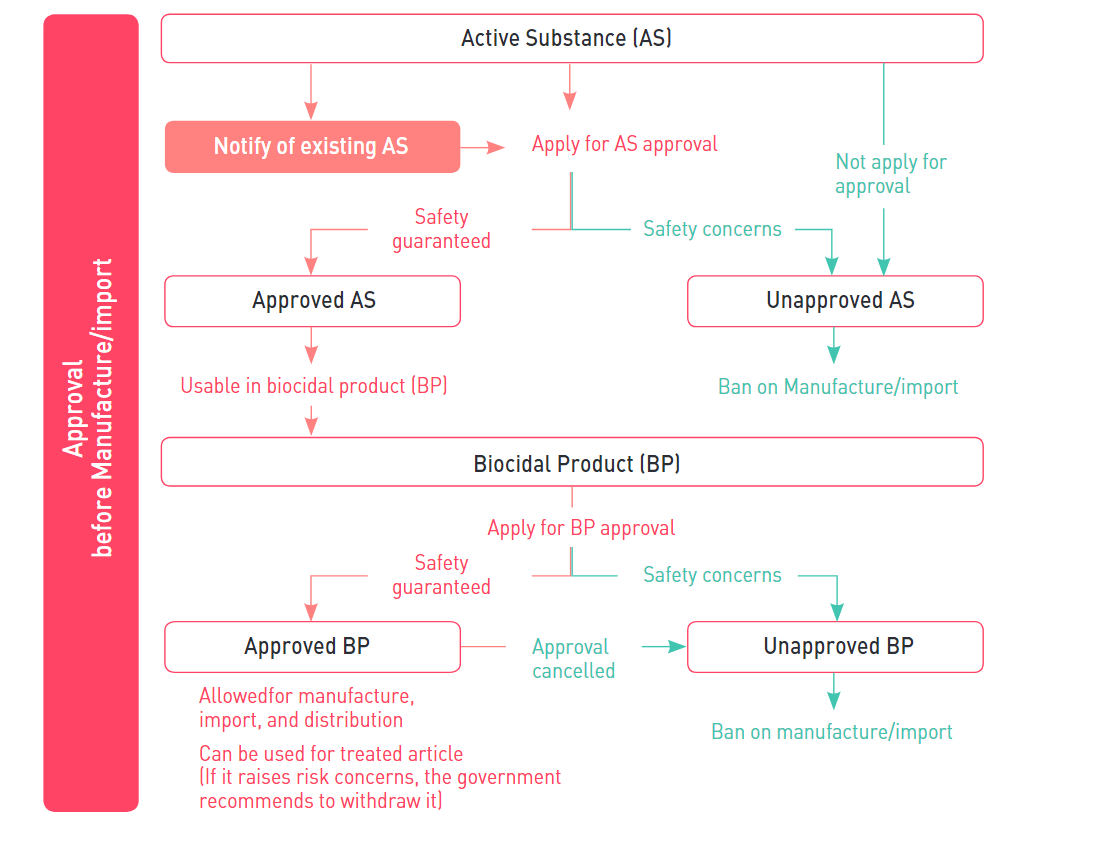
1. Notification of existing active substance
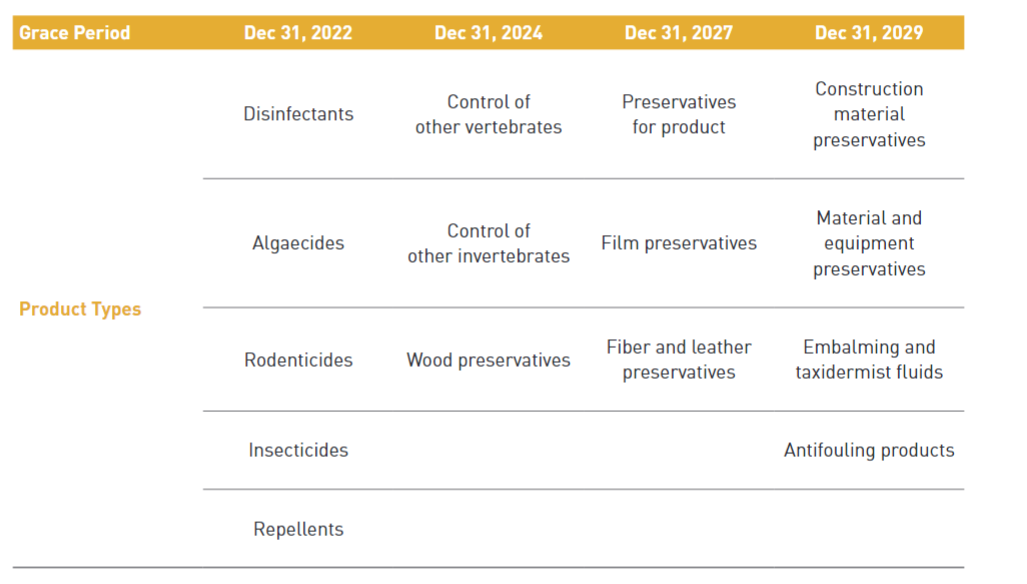
Any person who intends to manufacture or import an existing active substance which was contained in biocidal products distributed domestically until 31st December 2018 will be required to make a notification before manufacturing or importing to obtain a grace period of substance approval. When notifying the active substance, the product type of the substance must be declared together and a grace period of up to 10 years may be granted depending on the product type and hazard level. During the grace period, the existing AS can be manufactured or imported without AS approval.
2. Application Plan for AS Approval
Application plan for AS approval (a proposal on the procedure and method of preparing the substance approval application document) shall be submitted within one year from when the MOE Public Notice regarding the existing AS subject to grace period was publicly announced.
3. Approval of active substance
Any person who intends to manufacture or import an active substance shall get approved by the Ministry of Environment before manufacture or import such AS.
4. Approval of biocidal product
Similar to EU BPR, the Act requires that all biocidal products shall be approved before placing on the Korean market, and contained active substances must be approved prior to the approval of the biocidal product. The validity of biocidal product approval is set within the range of 10 years, and once it’s expired the person who intends to continue manufacturing and importing the product must get approval again. The person who obtained approval must have a manufacturing and storage facility that meets legal standards to maintain product quality as approved.
5. Treated article
Only approved biocidal products can be used in the treated article. If manufacturer claims that a treated article has biocidal properties, the manufacturer must label the treated article with information about biocidal products used and potential risks.
Record keeping and reporting
Importers or manufactures of Consumer Chemical Product Subject to Safety Check or biocides shall report the following information to the MoE every 2 years by 31st March.
Consumer Chemical Product Subject to Safety Check or biocidal products: name and volume of priority controlled substances and active substances contained in the products
Active substance: name and volume of Active substance.
Records of following information should be kept for a period of 10 years:
Name and quantity of Consumer Chemical Product Subject to Safety Check
Composition and mixing ratio contained in a consumer chemical product
Biocides Name, product name, and quantity
Composition and mixing ratio of active substances contained in a biocidal product
Name and content of biocidal products treated to article
Only Representative
If a foreign manufacturer or exporter does not provide any information of a chemical substance to an importer due to confidential business information (CBI), the importer cannot comply with K-BPR. To solve this CBI issue, foreign manufacturers can appoint a qualified only representative (OR) to fulfill the obligations of the importer under K-BPR.
CIRS K-BPR Service
Confirmation application for Consumer Chemical Products
Notification of existing active substances
Application Plan for AS Approval
Approval of active substance
Approval of biocidal product
- Review the labelling of treated article