The Inventory of Existing Chemical Substances in China (2013 edition) (IECSC) contains a total of 45,612 chemical substances, with 3,270 listed in the confidential part. The protection period for the identification information of substances in the confidential part is set to end on December 31, 2025.
On August 20, 2025, the Solid Waste and Chemicals Management Center (SCC) published the "Work Notice Concerning the Extension Application for Public Disclosure of Chemical Substance Identification Information" on its official website. According to the notice, any registration certificate holders that require an extension must prepare the necessary materials for explanation and submit their applications to the competent authority by September 15, 2025.
To assist relevant enterprises in understanding and complying with the extension application procedures, CIRS Group has compiled this handbook, which covers a variety of information, including:
- The confidentiality periods for chemical identification information listed in the Inventory of Existing Chemical Substances in China (IECSC);
- The conditions for applying for an extension;
- Details of the application materials;
- The online registration system operation procedures;
- The review criteria of the competent authority, and
- Professional guidance for registration certificate holders handling extension applications.
1. What are the rules for the confidentiality period of chemical identification information listed in the IECSC and the application of an extension?
- The Inventory of Existing Chemical Substances in China (2013 edition) (IECSC) contains a total of 45,612 chemical substances, with 3,270 listed in the confidential part. The protection period for the identification information of substances in the confidential part is set to end on December 31, 2025.
- Chemical substances that were manufactured, imported, sold, or processed/used in China before October 15, 2003, and were subsequently supplemented to the IECSC, must have their identification information fully publicly disclosed; application for information protection is not permitted.
- Chemical substances that obtained a registration certificate according to the former SEPA's Decree No. 17 were all added to the IECSC by June 18, 2021. For those still under identification information protection, the protection period is set to end on December 31, 2025, and no extension application for public disclosure is permitted.
- Chemical substances that received a regular registration certificate under the former MEP Order 7 before January 1, 2021, and for which the first activity has commenced, have been supplemented to the IECSC by the competent authority. This supplementation occurs in batches, five years after the substance's first activity. Among these, those approved for confidentiality during their registration are automatically listed in the confidential part. Their confidentiality period is set to expire on December 31, 2025. Applicants may apply to the competent authority by September 15, 2025, for an extension of public disclosure, for a maximum period not exceeding five years.
- Chemical substances that obtained a regular registration certificate under the former MEP Order 7 before January 1, 2021, but for which the first activity has not commenced, will be supplemented to the IECSC by the competent authority on December 31, 2025. Among these, those approved for confidentiality during registration may apply to the competent authority by September 15, 2025, for an extension of public disclosure, for a maximum period not exceeding five years.
- Chemical substances that obtained a regular registration certificate under the former MEP Order 7 during the regulatory transitional period (January 1, 2021 – June 30, 2021), will be supplemented in the IECSC by the competent authority five years after the date of initial registration (at the latest by June 30, 2026). Among these, those approved for confidentiality during registration may apply to the competent authority by December 31, 2025, for an extension of public disclosure, for a maximum period not exceeding five years.
- Chemical substances that obtained a regular registration certificate under the MEE Order 12, will be supplemented in the IECSC by the competent authority five years after the date of initial registration. Among these, those approved for confidentiality during registration may apply to the competent authority for an extension of public disclosure six months before the expiration of the confidentiality period, for a maximum period not exceeding five years.
- Chemical substances whose registration certificate holders voluntarily cancel their regular registration certificates, will still be supplemented in the IECSC by the competent authority five years after the date of initial registration. However, chemical substances whose regular registrations are withdrawn or revoked by the competent authority according to law will not be included in the IECSC.
- Chemical substances that obtained a simplified registration certificate or completed scientific research filing under the former MEP Order 7, and chemical substances that obtained a simplified registration certificate or completed filing under the MEE Order 12, are not included in the IECSC.
- If chemical substance identification information protection was not applied for or was not approved during the new chemical substance registration, an extension for the public disclosure of chemical identification information cannot be applied for.
2. How can we prepare for the application materials for the extension of public disclosure of chemical identification information?
The application materials should cover the following information:
- Prepare the basic information of the applicant. This includes the registration certificate number (including the certificate), name of the applicant and agent, contact details, and the name of the signatory (If the signatory is not the legal representative, a power of attorney from the legal representative is also required).
- Assess the basic situation of the applied substance. Whether the substance is a high-hazard substance, whether it poses significant potential environmental or health risks, where delaying disclosure could significantly impact public health or environmental interests.
- Review the applicant's information protection status. Clarify whether the applicant applied for the chemical identification information protection (e.g., chemical name) during registration and whether it was approved.
- Prepare necessary materials for extending the disclosure of identification information, including:
- The specific identification information fields for which extension is requested (e.g., Chinese/English chemical name, CAS No., molecular weight, molecular formula, structural formula, and SMILES code) and the requested extension period.
- Materials explaining whether the identification information subject to the extension request is known to the public (e.g., Is it available for the public or competitors to potentially learn this information from public sources or to associate it with the application company? Has this identification information been published or disclosed by domestic or foreign authorities?).
- Explanation of the commercial value of the identification information for which extension is requested, preferably supported by relevant evidence.
- Continuous precautionary measures taken by the applicant to safeguard this chemical identification information, providing details of these measures.
- The necessary clarification materials must be signed and stamped and submitted as an attachment.
3. Operational process for submitting the public disclosure extension application via the New Chemical Substance Online Registration System
To start, log in to the Ministry of Ecology and Environment (MEE) Online Service Hall (https://www.mee.gov.cn/), access the New Chemical Substance Online Registration System, and select the "Chemical Substance Identification Information Public Disclosure Extension Application Form" under the regular registration section.
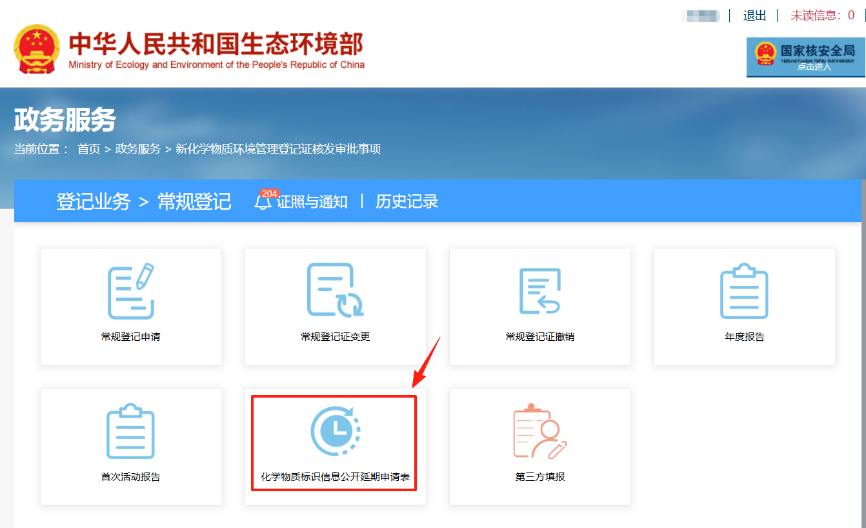
Click to enter the application form. Fill in each item step-by-step and upload relevant attachments, including the registration certificate and the necessary clarification materials for information protection. Items marked with an asterisk (*) are mandatory. It is recommended to periodically save your progress.
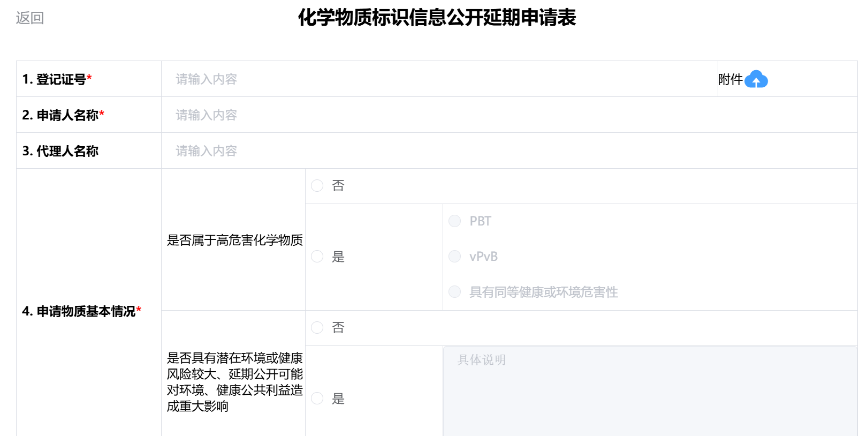
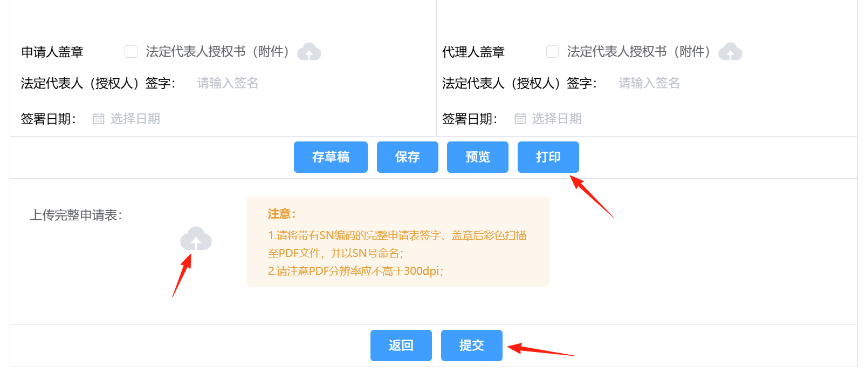
After completing the application form, it is advisable to preview it to confirm everything is correct before printing. (Note: after printing, the content of the application form cannot be edited or modified.) After printing, the form must be signed by the authorized signatory and stamped with the company seal. Then, upload a color scanned copy (PDF file) of the complete, signed, and stamped application form to the registration system. Click the Submit button to complete the application.
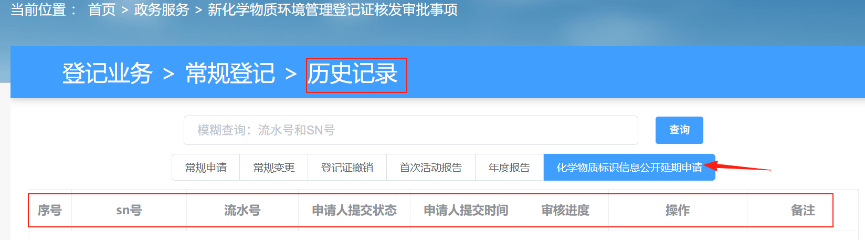
After submitting the application, applicants can view all submitted application records and their review status in the "History" section of the registration system.
4. Review decision from the competent authority on the extension application
Extension applications for substance information disclosure will not be approved in one of the following situations:
(1) The chemical substance has multiple registration certificate holders, and one or more of them did not submit the extension application on time.
(2) Upon review, the clarifications for the extension are deemed insufficient.
(3) The substance is of high hazard, or has significant potential environmental or health risks, and delaying disclosure could significantly impact public health or environmental interests.
The extension application will be approved if all registration certificate holders for the chemical substance submit their applications on time, the clarification for the extension is deemed sufficient and reasonable upon review, and the situation does not fall under item (3) above.
5. CIRS recommendations on the submission of extension application materials
(1) Did your company obtain any regular registration certificates under the former MEP Order 7?
- If No, your company does not need to submit extension applications.
- If Yes, proceed to the next question.
(2) Was the chemical substance approved for identification information confidentiality during the regular registration under MEP Order 7?
- If No, your company does not need to submit extension applications.
- If Yes, proceed to the next question.
(3) Does your company wish the substance's identification information to remain confidential in the IECSC after December 31, 2025?
- If No, your company does not need to submit extension applications.
- If Yes, your company needs to actively prepare application materials for extending the disclosure of identification information and submit the application via the New Chemical Substance Registration System before September 15, 2025.
(4) What aspects require special attention when preparing the application materials for extending the disclosure of identification information?
- Accurately assess the hazard of the applied substance. If the chemical substance is classified as a high-hazard chemical substance under MEE Order 12, or if it has health or environmental hazards of concern and potential for frequent human or environmental exposure, the extension application for this chemical substance will not be approved.
- Verify if the chemical identification information can be obtained from public sources. Public sources include published literature, public patents/documents, databases, search engines, official inventories, or publicly available data. If the chemical identification information can be retrieved from the public channels, it will be difficult to obtain approval for continued confidentiality based on our experience.
- Gather sufficient and reasonable supporting evidence demonstrating the commercial value of the chemical identification information. This includes, but is not limited to: Is the applicant the sole developer, holding exclusive patents for the substance? Does the substance have a clear technological leading advantage in its application field, with significant market potential and economic benefits? How critical is the substance for maintaining the company's high market share, leading industry position, and competitive advantage? What specific losses (and their extent) would occur if competitors learned this identification information? It is recommended to quantify the commercial value monetarily or numerically as much as possible and provide relevant evidentiary materials.
Finally, it is worth noting that the requirements for the extension application materials are largely consistent with those for the information protection application during new substance registration. However, since the implementation of MEE Order 12 on January 1, 2021, the competent authority's review of chemical identification information protection applications has been very strict, with only about 10% of new substances under regular and simplified registration being approved for identification information protection.
CIRS Group recommends that if your company needs to apply for an extension of the public disclosure of chemical identification information, please actively prepare the application materials according to the requirements, to ensure the necessary clarification materials are sufficient, reasonable, and supported by clear evidence.
Author

Bruce Wang – Deputy Technical Director of Chemical Technology Department, DCST, CIRS China
Mr. Bruce Wang, who received his master's degree in polymer chemistry and physics, is now the Deputy Technical Director of the Chemical Technology Department at CIRS. He is a very experienced chemical regulatory project leader with over ten years’ experience working in chemical risk assessment and new chemical substance registration in China. His professional team has completed more than 150 certificates for typical notification of new substances, as well as more than 1,200 certificates for simplified notification. In addition, he has a store of experience in identification of hazardous chemicals and China GHS.
Contact Us
Hangzhou REACH Technology Group Co., Ltd.
Tel: +86 571 87206574
Email: service@cirs-group.com

