Reusable medical devices should be designed for user-friendly and efficient cleaning and necessary disinfection or sterilization. Manufacturers of medical devices should adequately specify information on cleaning, disinfection or sterilization. At the same time, the manufacturer should keep the verification record of the relevant reprocessing information in the system documents in accordance with the requirements of The Good Manufacturing Practice for Medical Devices, to prove that the reprocessing information has been verified and is easy for users to understand and operable.
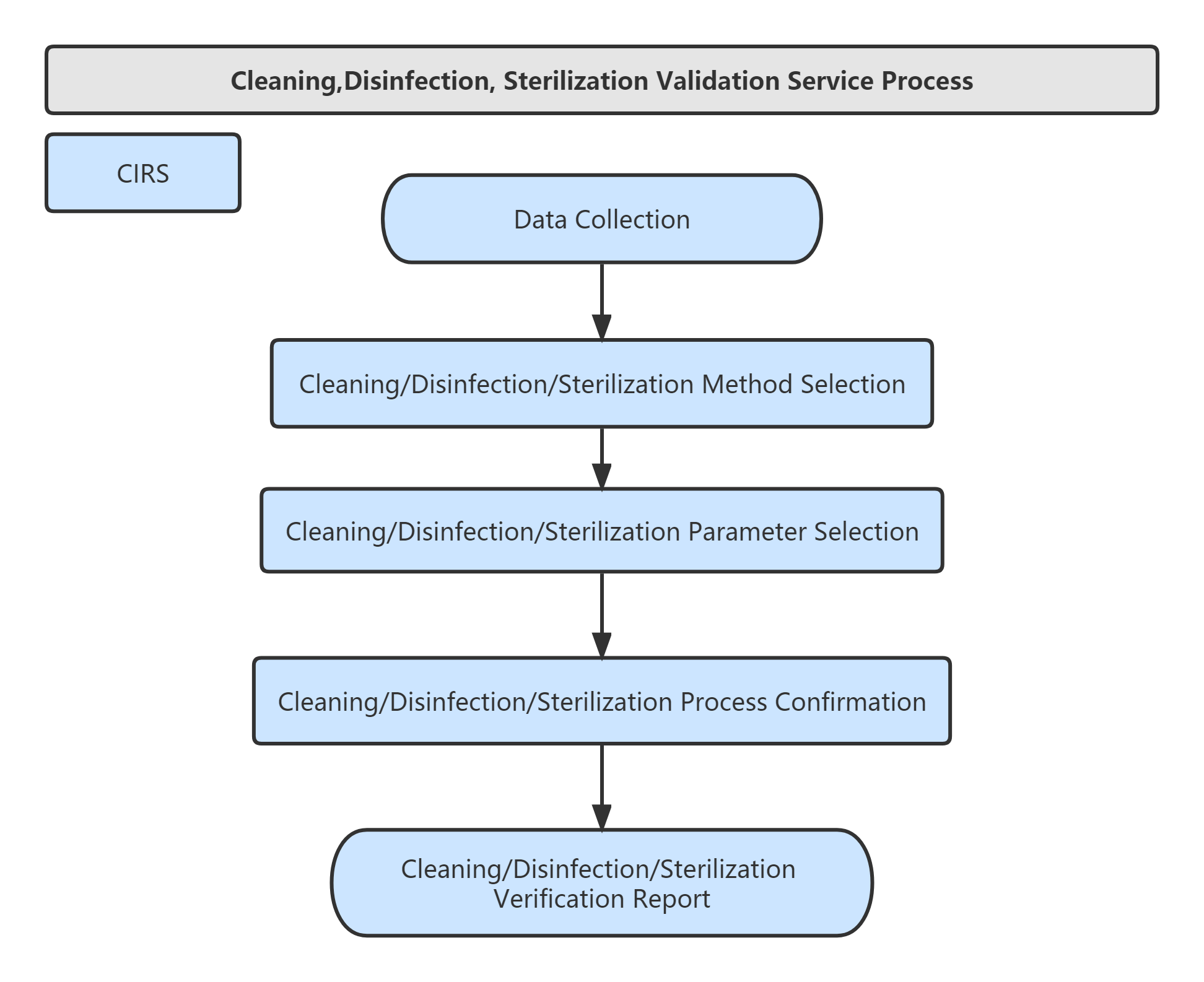
Validation Services of Cleaning,Disinfection, Sterilization:
- ISO17664-Processing of health care products-Information to be provided by the medical device manufacturer for the processing of medical devices
- ISO17665-1-Sterilization of health care products-Moist heat-Part 1:
- Requirements for the development, validation and routine control of a sterilization process for medical devices
- AAMI TIR12-Designing, Testing, And Labeling Medical Devices Intended For Processing By Health Care Facilities: A Guide For Device Manufacturers
- Hydrogen peroxide low temperature plasma sterilization verification
- Other verification
Service Process: