How to identify the class of medical device will be paid highly attention by the registrant. The main method to confirm the classification of medical device to classification catalogue and classification principle of medical device, application of medical device classification definition.
OUR SERVICE
CIRS professional team provides following classification determination service to be the foundation for medical device recording/registration procedure.
| Service Content | Advantage of CIRS | Service Details | |
| Determining the Classification | Identify the class of medical device according to CFDA published classification catalogue and classification principle of medical device. |
| Period: 8 business days Charge: free |
| Application of classification definition | This type of service is applicable to products that are unable to determine the type through the existing regulations, and need to submit classification definition applications to the CFDA | Data compilation: 30 working days Audit in the Provincial FDA: 1-2 months Audit in CFDA: 6-12 months Charge: according to the product | |
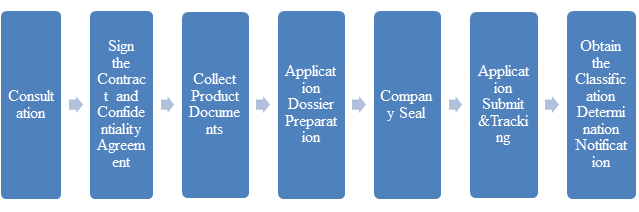
SERVICE FLOW CHART(Click on the picture to zoom in)
OUR TEAM
CIRS medical device service team mainly consists of experienced registration and clinical experts with master’s degree and PhD in medical background. Medical devices service team is divided into three professional groups: regulatory affairs service group, clinical trials service group and customs service group. Also we have good network with other professional institutions. And our goal is to provide the most professional, efficient, long- term and comprehensive service for our clients.
* If you have any comments or questions, please contact us at md@cirs-group.com.

