With the outbreak of this epidemic (COVID-19), the demand for medical devices for epidemic prevention has become very large. As one of the world's major suppliers, China has attracted many buyers.
In order to make it easier for buyers to make inquiries more quickly and conveniently, and avoid buying unregistered medical device products, you can find the registration information of the 7 categories of medical device products required for epidemic prevention at the following website.(Including new coronavirus detection reagents, ventilator, medical protective clothing, medical protective masks, medical surgical masks, disposable medical masks, infrared thermometers, etc.)
Query guide
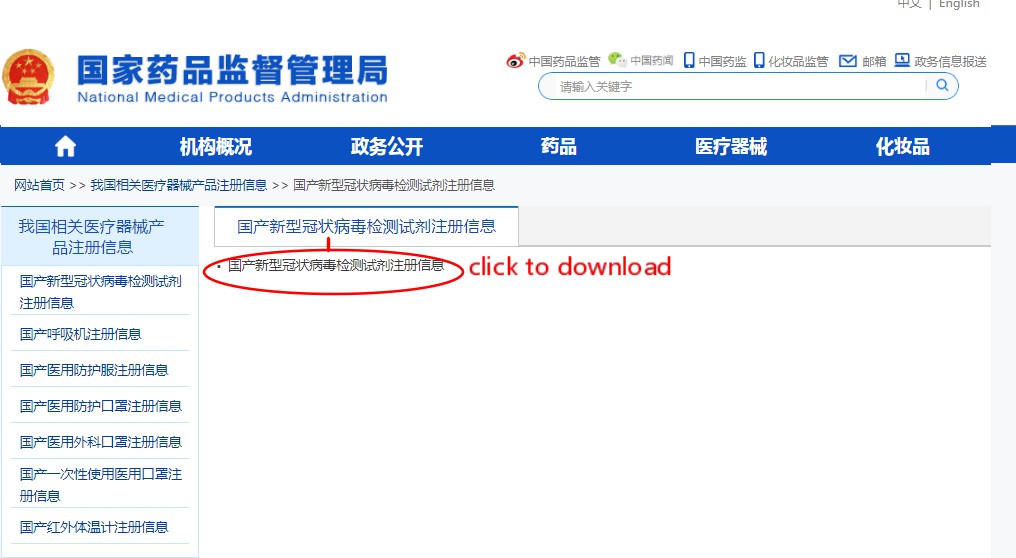
Step 1: click the link http://english.nmpa.gov.cn/
Step 2: Click the picture on the left side of the page”Medical Device Registration Information in China”
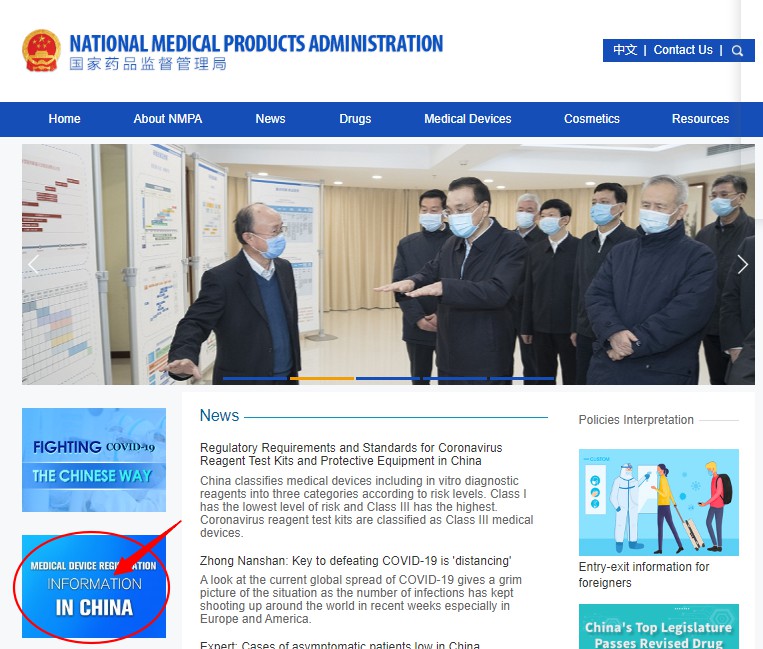
Step 3: Click on the medical device product type you want to query
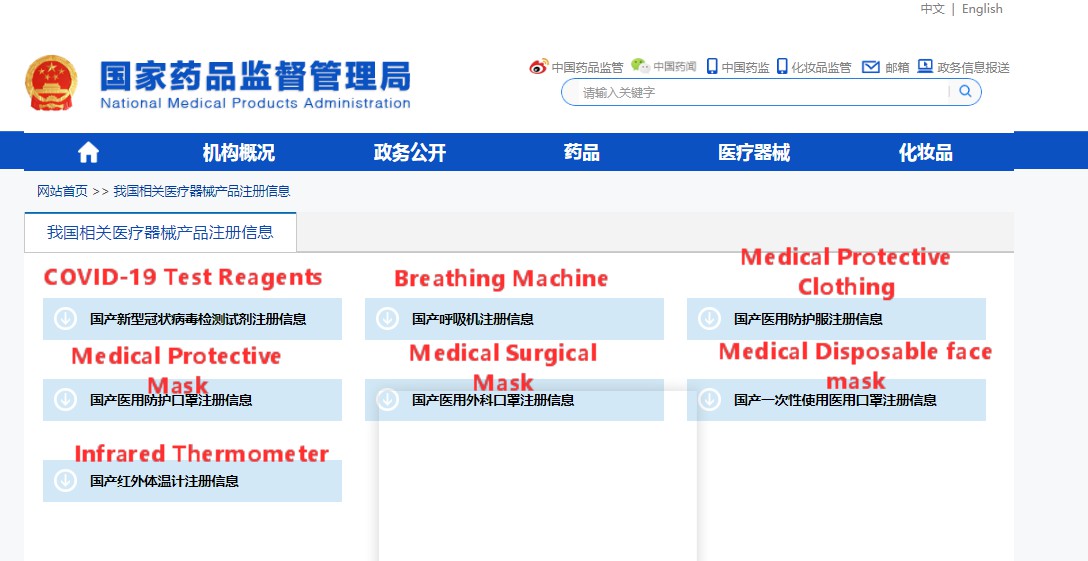
Step 4: Click and download the summary of registration information for this type of medical device product