In order to complete medical device information management, improve regulation’s efficacy, realize resource sharing of record information, CFDA has developed Medical device registration management information system-subsystem and put into use on Sep.10, 2015. The enterprise side of the subsystem is used for record of class I, information search, statistical analysis, and so on.
Formerly applicant just submits the paper application documents to record units. Now, applicant shall fill the Record Form online and print it, then submit the record form and relevant documents to record units.
How to use the subsystem? Now, we will introduce the operating steps for record by word and screenshot of the page.
1. User registration
If you use the system for the first time, you shall register at first.
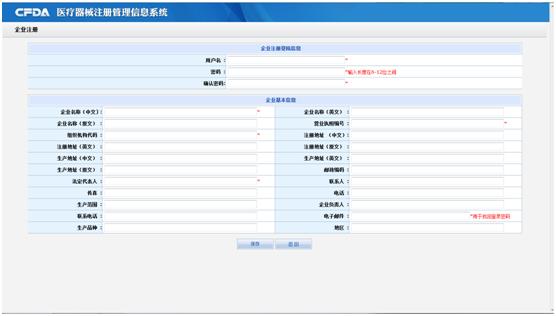
Clicking the button of User Registration, you will enter the registration screen (as following), you must filling the information (* means the field is a required).
2. Record application
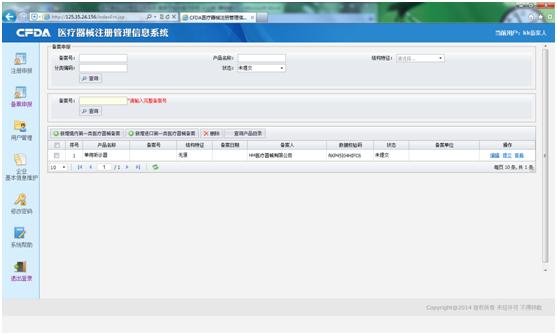
Enter the subsystem through the log-in screen, you will get the record application screen (as following). According to your product (imported or domestic), you shall choice which service you need (see red square), if your product is domestic, you shall click the left one (新增境內第一類醫療器械備案), otherwise, the right one.
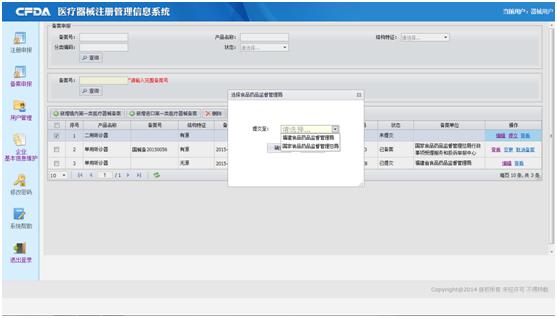
With domestic product, for example, click the left button ((新增境內第一類醫療器械備案), you will get the Record Form and to fill it. Please read the instruction before filling. After filling the form, you can submit the form to your local Food and Drug administration. If the product comes from Taiwan, you shall submit to the CFDA or Fujian Food and Drug administration. If your product is imported, it will be submitted to CFDA directly after submitting.
Un-submitted form, the applicant can compile and delete it. Submitted but un-received form, the applicant can compile, but not delete. Submitted and received form, the applicant cannot compile and delete.
If you want more information about Change Record, information search, and so on, please feel free to contact us.
Formerly applicant just submits the paper application documents to record units. Now, applicant shall fill the Record Form online and print it, then submit the record form and relevant documents to record units.
How to use the subsystem? Now, we will introduce the operating steps for record by word and screenshot of the page.
1. User registration
If you use the system for the first time, you shall register at first.
Clicking the button of User Registration, you will enter the registration screen (as following), you must filling the information (* means the field is a required).
2. Record application
Enter the subsystem through the log-in screen, you will get the record application screen (as following). According to your product (imported or domestic), you shall choice which service you need (see red square), if your product is domestic, you shall click the left one (新增境內第一類醫療器械備案), otherwise, the right one.
With domestic product, for example, click the left button ((新增境內第一類醫療器械備案), you will get the Record Form and to fill it. Please read the instruction before filling. After filling the form, you can submit the form to your local Food and Drug administration. If the product comes from Taiwan, you shall submit to the CFDA or Fujian Food and Drug administration. If your product is imported, it will be submitted to CFDA directly after submitting.
Un-submitted form, the applicant can compile and delete it. Submitted but un-received form, the applicant can compile, but not delete. Submitted and received form, the applicant cannot compile and delete.
If you want more information about Change Record, information search, and so on, please feel free to contact us.

