Rare diseases are those with very low incidence. Rare diseases are also known as " Orphan disease ", according to the definition of the World Health Organization (WHO), the number of patients with rare diseases accounts for 0.65 ‰ to 1 ‰ of the total population. The exact cause of the rare disease is still remain unknown, mostly congenital diseases. Due to the low incidence of rare diseases, the related research resources are also very limited. Therefore, the current drugs and medical devices for the prevention and treatment of rare diseases are quite limited.
The medical device for the prevention and treatment of rare diseases is a special medical device. The National Medical Products Administration (former CFDA) has also issued medical device regulations and guidelines related to the diagnosis and treatment of rare diseases.To speed up the management of the registration of medical devices for rare diseases, further improve the quality of registration examinations and encourage the development of medical devices for rare diseases. On October 22, 2018, in order to implement the "Opinions on Deepening the Reform of the Examination and Approval System and Encouraging the Innovation of Drug Medical Devices" by the General Office of the State Council (Tingzi [2017] No. 42), the National Medical Products Administration (former CFDA) promulgated the “Notice of the State Drug Administration on Issuing the Guiding Principles for the Registration Review of Medical Devices for the Control of Rare Diseases”(No. 101 of 2018). Below is the interpretation from CIRS on the new guiding principle:
1. What are the common rare diseases and medical devices for the prevention and treatment of rare diseases?
At present, it is more common in albinism, acromegaly, idiopathic pulmonary hypertension, phenylketonuria, mitochondrial disease and so on. The first batch of 121 rare disease catalogues jointly published by the National Health and Health Commission, the Ministry of Science and Technology, the Ministry of Industry and Information Technology, the National Medical Products Administration (former CFDA), and the State Administration of Traditional Chinese Medicine on May 22, 2018 (For specific information, please see appendix). At present, the domestic medical devices for the prevention and treatment of rare diseases are mainly for the detection of proteins related to rare diseases, such as the aquaporin 4 antibody assay kit (enzyme-linked immunosorbent assay) and the gene sequence for detecting rare disease genes.
2. Rare disease prevention and treatment medical device registration requirements
From January 1st, 2017, the National Medical Products Administration officially implemented the “Priority Approval Procedure for Medical Devices”. The registration of medical devices for the prevention and treatment of rare diseases can enter the priority approval process, and the following corresponding information should be provided:
- The incidence data and related supporting data of the indications for the product
;- Supporting documents of indication from the rare disease
- Summary of the clinical status of the indications;
- Obvious clinical advantages and related supporting data of the product compared with existing products or treatment methods.
For the projects that are given priority for approval, the National Medical Products Administration will speed up the review and approval of the whole process, prioritize technical review, prioritize the verification of medical device registration quality management system, prioritize administrative approval, shorten time to market, and ensure the corresponding results and products can be applied to clinical use as soon as possible.
3. Preclinical trial
Rare disease prevention and treatment medical device require preclinical testing to verify their effectiveness and to fully assess their risk range. Preclinical tests should follow the following principles:
? Provide detailed research background information on the rare diseases that are prevented by the declared products, including the causes of the disease, clinical symptoms, epidemiological characteristics, and the diagnosis and effective treatment methods of the rare diseases, and clarify the advantages and disadvantages of the existing methods. The research data may be the result of the applicant's scientific research or related literature.
? Fully elaborate the mechanism of action of the declared product, clarify the potential risks of the declared product, and conduct a sufficient pre-clinical evaluation. The pre-clinical study of the product should be able to confirm that the product risk is within the acceptable range.
? Provide detailed research data of the declared products, and recommend simulation tests in the product performance research process to verify the performance of the products under simulated conditions, and demonstrate the rationality of the simulation parameters. Conduct appropriate cell and animal tests if necessary. Product research data should be able to demonstrate the possible effectiveness of the product
? Provide sufficient comparative research data on the declared products with existing diagnostic and therapeutic methods (if any) and similar products (if any) already listed, and demonstrate the product advantages and patient benefits clearly.
4. Clinical trial requirements
4.1. Rare disease prevention and treatment medical device that required to have clinical trial
Medical devices used for the prevention and treatment of rare diseases, if their preclinical studies cannot prove that the clinical application of the product patients benefit significantly greater than the risk, clinical trials will be required.
4.2. Clinical trial requirements
(I)Medical device for treating rare diseases
For rare diseases that do not have effective treatment methods currently, the declared products and the basis for the formulation should be clearly defined in the clinical trials; for rare diseases that currently have effective treatments, the declared products shall provide comparative study of the effectiveness of existing treatments and products, and patient risk benefit ratios can be aggregated from clinical historical study data.
(II)Medical device for diagnosing rare diseases
For the diagnosis of rare diseases or auxiliary diagnosis, the main evaluation indicators of clinical trials are clinical sensitivity and clinical specificity. The comparison method in clinical trials can be the recognized diagnostic standard or the similar products already on the market, and results should be follow up if necessary. Products used for rare disease screening should be based on reasonable clinical evaluation indicators. The method used to confirm screening results in clinical trials should be a clinically accepted diagnostic criteria. Screening results should be followed up or confirmed by other methods if necessary
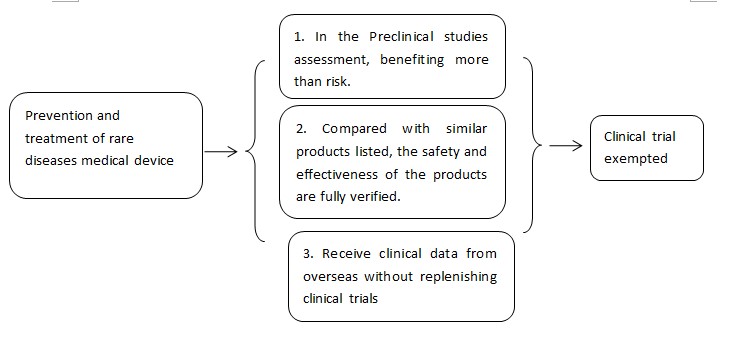
5. Rare disease prevention and treatment medical device exempted from clinical trial
5.1 Basic principles of clinical trials exemption
(I)For medical devices used for the treatment of rare diseases, the pre-clinical research or other evidence can determine that the patient's benefit from using the device is significantly greater than the risk, and the company is in accordance with the technical review on the premise of communication with the technical review department. Under the advice from technical review department, clinical trials can be exempted.
(II)For medical devices (such as in vitro diagnostic reagents) that have been marketed in the same category, the clinical application of the same type of product can be safely and effectively evaluated; for in vitro diagnostic reagent products that are exempted of clinical trials,the comparison method of the same varieties shall confirmed the performance of the clinical samples. The safety and effectiveness of the same variety of products selected in the above evaluation process has been fully verified.
(III)For medical devices used for the treatment of rare diseases that already marketed in other countries, if the clinical trial data from oversea meets the ‘Accept technical guidelines for overseas clinical trial data of medical devices’, they could be used as clinical trial submission, If the review department in the technical review process does not need to supplement the domestic clinical trial before the product is listed, it can be exempted from clinical trials.
5.2、 Process of clinical trial exemption
Appendix 1: First batch of rare disease list
No. | Name |
1 | 21-Hydroxylase Deficiency |
2 | Albinism |
3 | Alport Syndrome |
4 | Amyotrophic Lateral Sclerosis |
5 | Angelman Syndrome |
6 | Arginase Deficiency |
7 | Asphyxiating Thoracic Dystrophy (Jeune Syndrome) |
8 | Atypical Hemolytic Uremic Syndrome |
9 | Autoimmune Encephalitis |
10 | Autoimmune Hypophysitis |
11 | Autoimmune Insulin Receptopathy (Type B insulin resistance) |
12 | Beta-ketothiolase Deficiency |
13 | Biotinidase Deficiency |
14 | Cardic Ion Channelopathies |
15 | Carnitine Deficiency |
16 | Castleman Disease |
17 | Charcot-Marie-Tooth Disease |
18 | Citrullinemia |
19 | Congenital Adrenal Hypoplasia |
20 | Congenital Hyperinsulinemic Hypoglycemia |
21 | Congenital Myasthenic Syndrome |
22 | Congenital Myotonia Syndrome (Non-Dystrophic Myotonia, NDM) |
23 | Congenital Scoliosis |
24 | Coronary Artery Ectasia |
25 | Diamond-Blackfan Anemia |
26 | Erdheim-Chester Disease |
27 | Fabry Disease |
28 | Familial Mediterranean Fever |
29 | Fanconi Anemia |
30 | Galactosemia |
31 | Gaucher’s Disease |
32 | Generalized Myasthenia Gravis |
33 | Gitelman Syndrome |
34 | Glutaric Acidemia Type I |
35 | Glycogen Storage Disease (Type I、II) |
36 | Hemophilia |
37 | Hepatolenticular Degeneration(Wilson Disease) |
38 | Hereditary Angioedema (HAE) |
39 | Hereditary Epidermolysis Bullosa |
40 | Hereditary Fructose Intolerance |
41 | Hereditary Hypomagnesemia |
42 | Hereditary Multi-infarct Dementia (Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy, CADASIL) |
43 | Hereditary Spastic Paraplegia |
44 | Holocarboxylase Synthetase Deficiency |
45 | Homocysteinemia |
46 | Homozygous Hypercholesterolemia |
47 | Huntington Disease |
48 | Hyperornithinaemia-Hyperammonaemia-Homocitrullinuria Syndrome |
49 | Hyperphenylalaninemia |
50 | Hypophosphatasia |
51 | Hypophosphatemic Rickets |
52 | Idiopathic Cardiomyopathy |
53 | Idiopathic Hypogonadotropic Hypogonadism |
54 | Idiopathic Pulmonary Arterial Hypertension |
55 | Idiopathic Pulmonary Fibrosis |
56 | IgG4 related Disease |
57 | Inborn Errors of Bile Acid Synthesis |
58 | Isovaleric Acidemia |
59 | Kallmann Syndrome |
60 | Langerhans Cell Histiocytosis |
61 | Laron Syndrome |
62 | Leber Hereditary Optic Neuropathy |
63 | Long Chain 3-hydroxyacyl-CoA Dehydrogenase Deficiency |
64 | Lymphangioleiomyomatosis (LAM) |
65 | Lysinuric Protein Intolerance |
66 | Lysosomal Acid Lipase Deficiency |
67 | Maple Syrup Urine Disease |
68 | Marfan Syndrome |
69 | McCune-Albright Syndrome |
70 | Medium Chain Acyl-CoA Dehydrogenase Deficiency |
71 | Methylmalonic Academia |
72 | Mitochodrial Encephalomyopathy |
73 | Mucopolysaccharidosis |
74 | Multifocal Motor Neuropathy |
75 | Multiple Acyl-CoA Dehydrogenase Deficiency |
76 | Multiple Sclerosis |
77 | Multiple System Atrophy |
78 | Myotonic Dystrophy |
79 | N-acetylglutamate Synthase Deficiency |
80 | Neonatal Diabetes Mellitus |
81 | Neuromyelitis Optica |
82 | Niemann-Pick Disease |
83 | Non-Syndromic Deafness |
84 | Noonan Syndrome |
85 | Ornithine Transcarbamylase Deficiency |
86 | Osteogenesis Imperfecta (Brittle Bone Disease) |
87 | Parkinson Disease (Young-onset , Early-onset) |
88 | Paroxysmal Nocturnal Hemoglobinuria |
89 | Peutz-Jeghers Syndrome |
90 | Phenylketonuria |
91 | POEMS Syndrome |
92 | Porphyria |
93 | Prader-Willi Syndrome |
94 | Primary Combined Immune Deficiency |
95 | Primary Hereditary Dystonia |
96 | Primary Light Chain Amyloidosis |
97 | Progressive Familial Intrahepatic Cholestasis |
98 | Progressive Muscular Dystrophy |
99 | Propionic Acidemia |
100 | Pulmonary Alveolar Proteinosis |
101 | Pulmonary Cystic Fibrosis |
102 | Retinitis Pigmentosa |
103 | Retinoblastoma |
104 | Severe Congenital Neutropenia |
105 | Severe Myoclonic Epilepsy in Infancy (Dravet Syndrome) |
106 | Sickle Cell Disease |
107 | Silver-Russell Syndrome |
108 | Sitosterolemia |
109 | Spinal and Bulbar Muscular Atrophy (Kennedy Disease) |
110 | Spinal Muscular Atrophy |
111 | Spinocerebellar Ataxia |
112 | Systemic Sclerosis |
113 | Tetrahydrobiopterin Deficiency |
114 | Tuberous Sclerosis Complex |
115 | Tyrosinemia |
116 | Very Long Chain Acyl-CoA Dehydrogenase Deficiency |
117 | Williams Syndrome |
118 | Wiskott-Aldrich Syndrome |
119 | X-linked Agammaglobulinemia |
120 | X-linked Adrenoleukodystrophy |
121 | X-linked Lymphoproliferative Disease |

