Confirm the Clinical Trial Requirements by Steps
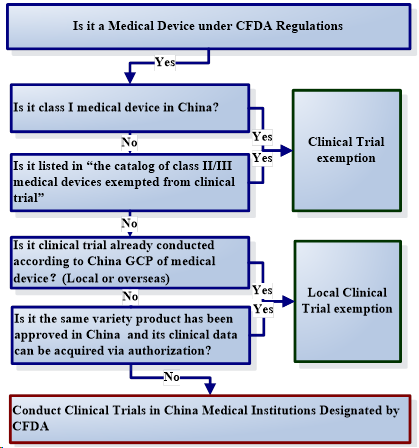
Which product can be exempted from clinical trial?
The class I medical device or the product which listed in “the catalogue of Class II & III medical devices exempted from clinical trials” can be exempted from Clinical Trials while applying for medical device initial registration. For the medical device exempt from clinical trial, they are only required to submit the comparison information with the product already approved in China by comparing the application relative information and detailed contents in the catalogue and the comparing
Other two conditions can be exempted from local clinical trials as follows:
- The product already conducted clinical trial according to China GCP of medical device (CFDA order No. 25 2016) or;
- The product whose same variety product’s clinical and technical information can be acquired via authorization.

