According to the "Regulations on the Supervision and Administration of Medical Devices" (No. 739), medical devices are classified and managed according to the degree of risk.
The Class II medical device is the medical device that is moderate-risk and requires strict control and management to ensure their safety and effectiveness. The Class III medical device is the medical device that is high-risk and requires special measures to strictly control management to ensure their safety and effectiveness.
Overseas medical device manufacturers need to entrust a domestic agent to register with the NMPA.
Regulation:
"Regulations on the Supervision and Administration of Medical Devices" (No. 739)
Service Process :
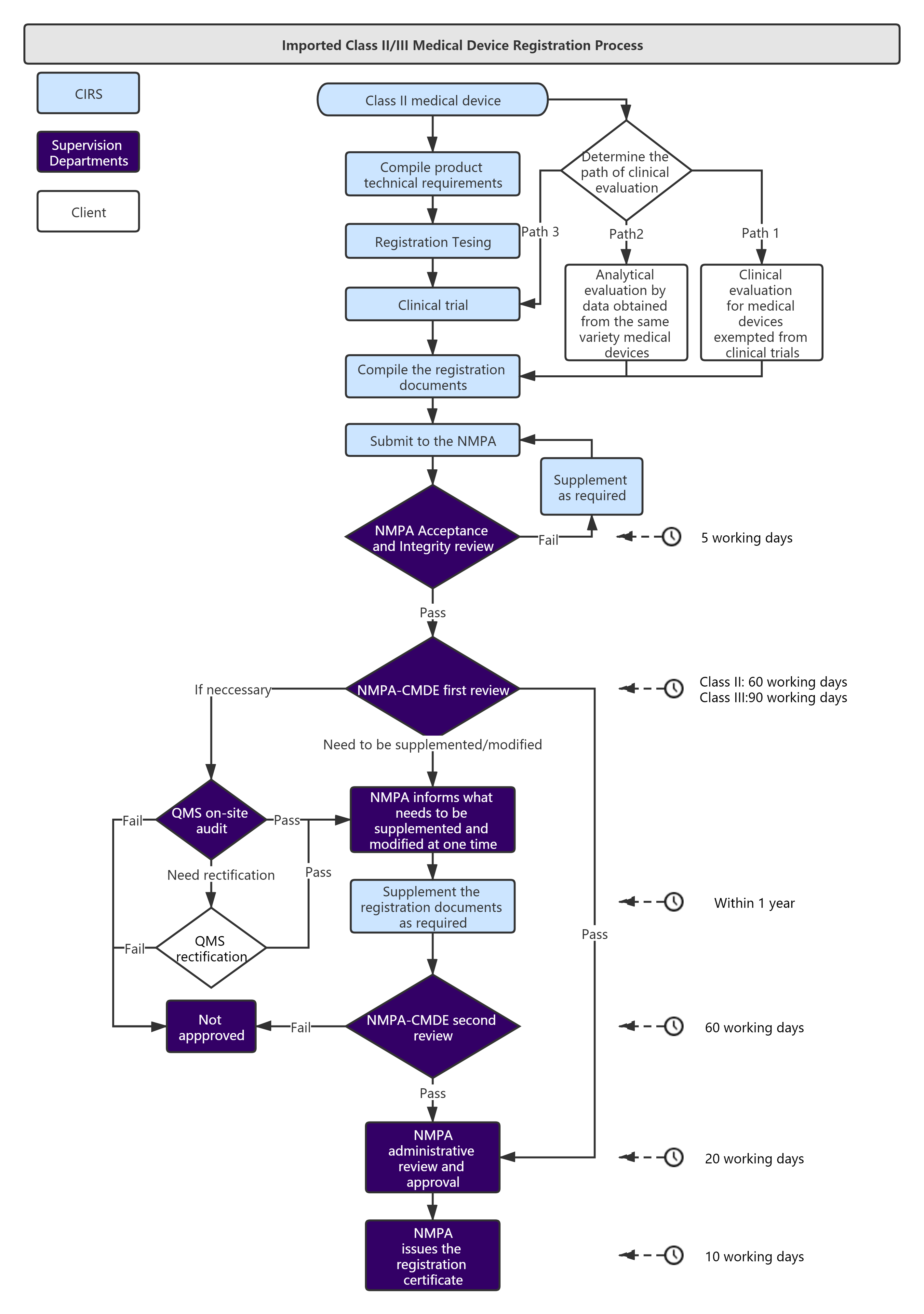
Administrative Fee:
Initial registration: RMB 210,900
Registration change: RMB 42,000
Registration renewal: RMB 40,800
Related Service:
Product classification determination
Class I medical device filing and filing change
Class II/III medical device registration, registration change and renewal
Product testing and rectification technical support
Technical files compilation
Medical device registration under the MAH system
Registration of imported-to-domestic products
Follow-up and correction of medical device registration technical review