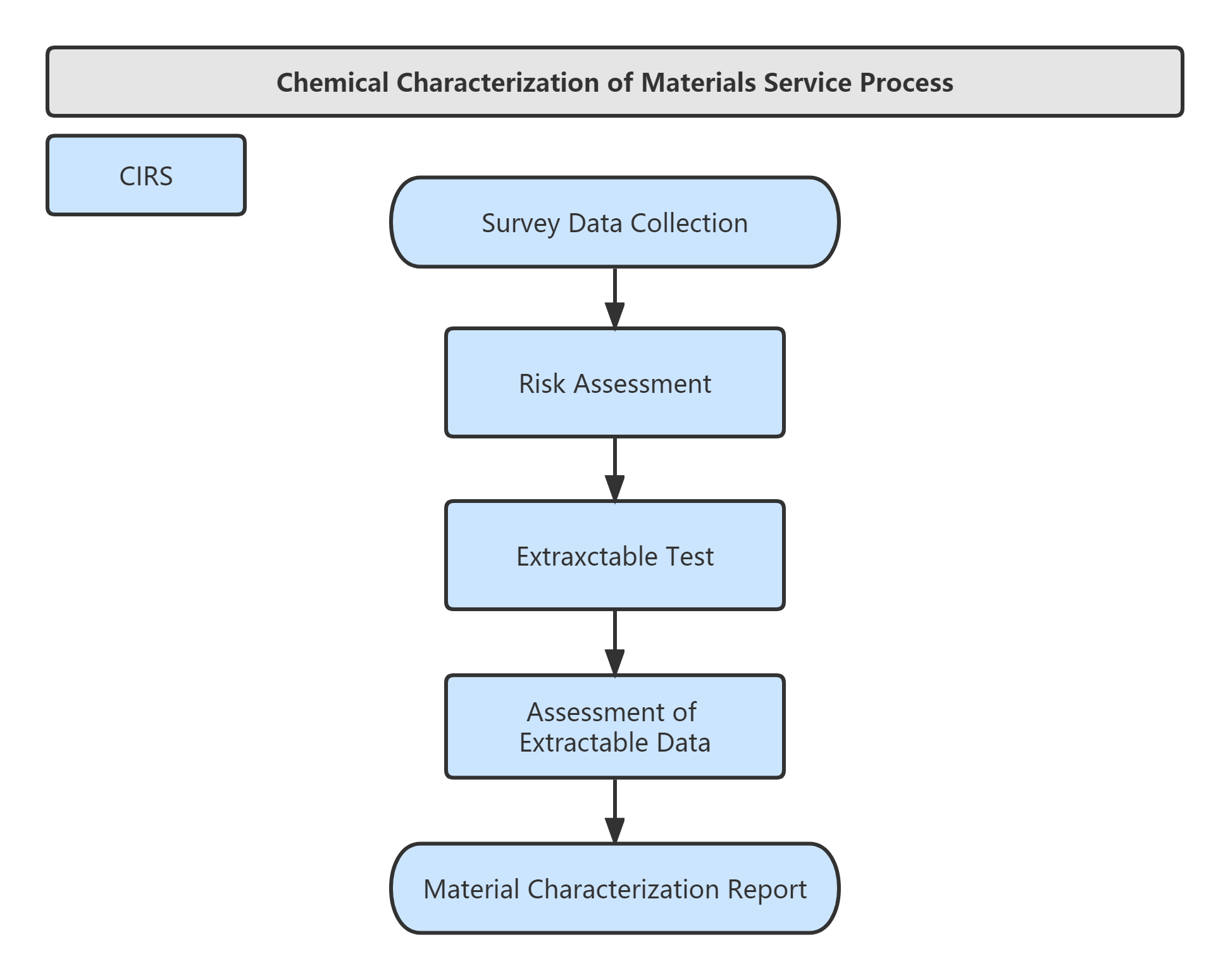
Standard ISO 10993-1 and GB/T16886.1 provide a framework for biological evaluation. With the development of scientific knowledge, biological evaluation system also needs to gradually transform from traditional biocompatibility tests to modern concepts based on risk assessment . During the risk assessment process, priority is given to the evaluation of chemical/physical properties and in vitro model tests.
Chemical characterization services of medical device materials:
- Identification of the materials of construction of the medical device
- Characterization of manufacturing materials through qualitative and quantitative chemical composition of materials
- Medical device characterization for chemical substances introduced during manufacturing process (e.g. processing aids, process contaminants, sterilization residues)
- Estimation of the possibility of releasing chemical substances from medical devices or their manufacturing materials under clinical condition of use
- Determination of chemical substances released by medical devices under clinical condition of use
Service Process: