Applicable Scope of Medical Devices
Data exchange, remote control and storage medium related class II or III medical devices registered in China, such as registration of wearable medical devices, app module
Definition of Medical Device Network Security
The medical device network security is deemed to ensure the medical device data confidentiality, integrity, availability, authenticity, accountability, non-repudiation and reliability.
Main Point of Network Safety Protection Safeguard Measures
Medical device network safety protection can be divided into product aspect and system aspect. And the safeguard measures include management measures, physical measures and technical measures. This guideline is focused on the technical safeguard measures of the product aspect
Network Safety Consideration
1. Data consideration
- Medical device data include: health data and devices data.
- Medical device data exchange methods: network and memory intermediaries.
3. Ready-made software consideration
4. Network safety documents
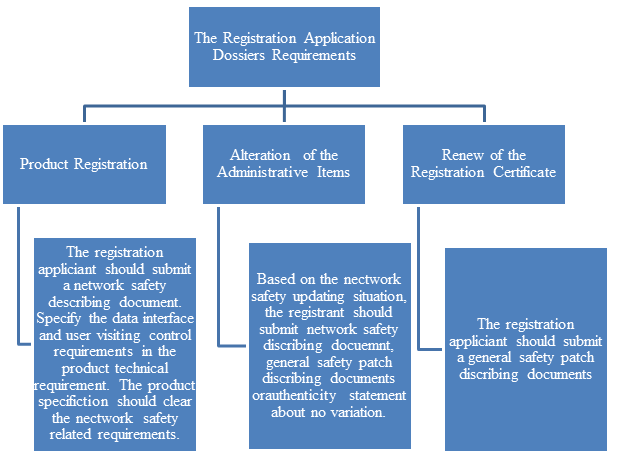
5. Registration application dossiers requirements (Click on the picture to zoom in)
*If you have any comments or questions, please contact us at md@cirs-group.com.

