1. Introduction
Product Name: nasal irrigator, nasal wash
Product Composition: consists of a main unit, a water tank, a nasal wash (adult and child type), and a power adapter.
Working Principle:
After the nasal irrigator is energized, the motor converts the electrical energy into mechanical pressure. The pressure is used to deliver warm water/physiological saline into the nostrils, through the nasal vestibule, sinus, and nasal passages around the nasopharynx, and from the other side of the nostrils. Through the above path, the sterilizing action of the physiological saline itself and the impact force of the water flow are used to discharge the accumulated dirt and harmful substances in the nasal cavity, thereby achieving the purpose of cleaning and daily care of the nasal cavity.
Typical Structure:
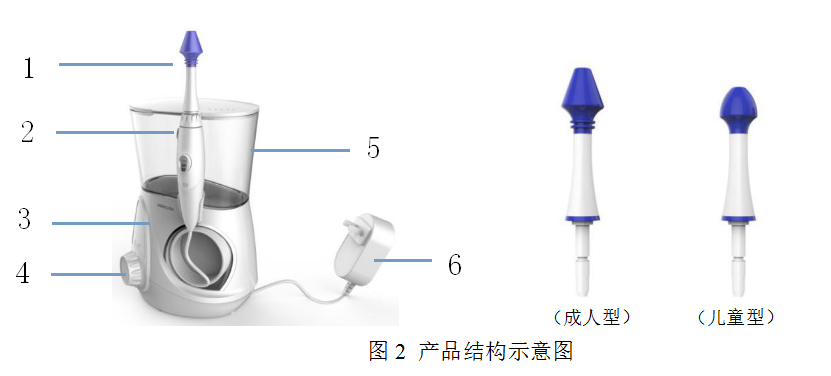
Adult type Child type
No. | Name | Description |
1 | Head | The outlet of the water flow controls the water volume stably and sprays evenly |
2 | Nozzle release button | Use and pause control buttons |
3 | Host | Control the operation of the nasal irrigator |
4 | Rotary switch | Adjustment circuit closure |
5 | Water tank | Water storage |
6 | Power adapter | Connected to the power supply |
Scope of application: For nasal irrigation and daily nasal hygiene
Applicable people: Suitable for adults and children with nasal cleansing needs
2. Classification
Classification Code: 14-07-01 Class II Flushing device
Product Description: Usually composed of a host, a heating module, and a power module
Expected Usage: For rinsing the natural cavity (excluding vaginal), rinsing the tissue during and after surgery, and warming the rinsing fluid
3. Registration Unit
1. Different principle
Passive polymer material nasal irrigators and active electric irrigators cannot be registered together.
2. Sterile and non-sterile
The polymer material type irrigator is a class II device provided by sterilizing, and a class I device provided by non-sterile; the electric nasal lavage device is class II.
3. Whether it contains drugs
Separate registration with medicinal ingredients and without drugs.
4. Technical Requirements
1. Appearance
The appearance of the nasal irrigator should be neat, smooth and uniform in color; no defects such as sharp edges, burrs, cracks, etc.; the characters and signs on the panel should be clearly visible; the installation and connection parts should be tight, the movable parts should be used, the rotation is flexible and reliable; the water tank is leak-free. .
2. Tank capacity
The water tank capacity is 700ml.
3. Flushing pressure
The water pressure at the outlet of the nasal wash should be between 0.1 MPa and 0.3 MPa.
4. Flushing flow rate
The water flow rate at the outlet of the nasal wash should be no more than 10 ml/s.
5. Flush height
The outlet height of the adult nasal wash outlet is 4.0 cm ± 1.0 cm; the height of the child's nasal wash outlet is 2.0 cm ± 1.0 cm.
6. Irrigator piping system effluent chemical properties
6.1 pH
The difference between the pH of the eluate and the blank control solution should be ≤1.0;
6.2 Heavy metal content
The heavy metal content of the eluate should be ≤1.0 μg/mL.
7. Electrical safety requirements
Should meet the requirements of GB9706.1-2007.
8. Electromagnetic compatibility requirements
Should meet the requirements specified in YY 0505-2012.
9. Environmental test requirements
It should meet the requirements of the climatic environment test group II and the mechanical environment group II in GB/T 14710-2009.
Table 1 Relevant Product Standards
Standard Number | Standard Name |
GB/T 191-2008 | Packaging storage and transportation icon |
YY/T 0316-2016 | Medical device risk management for medical devices |
YY/T 0466.1-2009 | Medical devices - Symbols for labeling, marking and providing information on medical devices - Part 1: General requirements |
GB/T 14710-2009 | Medical electrical environment requirements and test methods |
GB 9706.1-2007 | Medical electrical equipment - Part 1: General requirements for safety |
YY 0505-2012 | Medical electrical equipment - Part 1-2: Safety commons Parallel standard: Electromagnetic compatibility Requirements and testing |
GB/T 14233.1-2008 | Medical infusion, blood transfusion, injectables - Test methods - Part 1 |
YY(/T): Industry standard (Recommendation)
GB(/T): National standard (Recommendation)
5. Clinical Trials
Clinical Trials: required
6. Registration Cost and Duration
1) Product Testing
Test items | Testing duration | Testing fee(USD) |
Safety Performance | 60 working days | 3,000 |
EMC (Internal and external circuits ) | 90 working days | 4,500*2 |
Biocompatibility | 60 working days | 3,500 |
2) Technology Approval
60 working days
Another 60 working days if the 1-year supplement is required
3) Administrative Approval
20 working days
If you would like to get the detailed registration proposal, please contact us via md@cirs-group.com.

