In the wave of globalization in the food industry, innovation and safety are inseparable. An increasing number of companies are actively pursuing the US FDA Generally recognized as safe (GRAS) notice for new food ingredients. Unlike self-GRAS, the FDA GRAS requires the formation of GRAS evaluation materials, and when GRAS materials are submitted to the FDA, the FDA issues the inventory notices on its official website and periodically updates the information. The latest update to this inventory was on January 7, 2025.
CIRS Group has conducted a detailed statistical analysis and summary of substances submitted for FDA GRAS in 2024, aiming to provide clients with valuable insights.
1. Summary of the FDA GRAS notice in 2024
From 2024 until January 7, 2025, 13 substances received the letter stating “the FDA has no questions” (including two submitted in 2024 and 11 submitted before 2024). There are currently 55 substances that were submitted in 2024 and are still pending. Additionally, seven ceased to be evaluated (three were resubmitted).
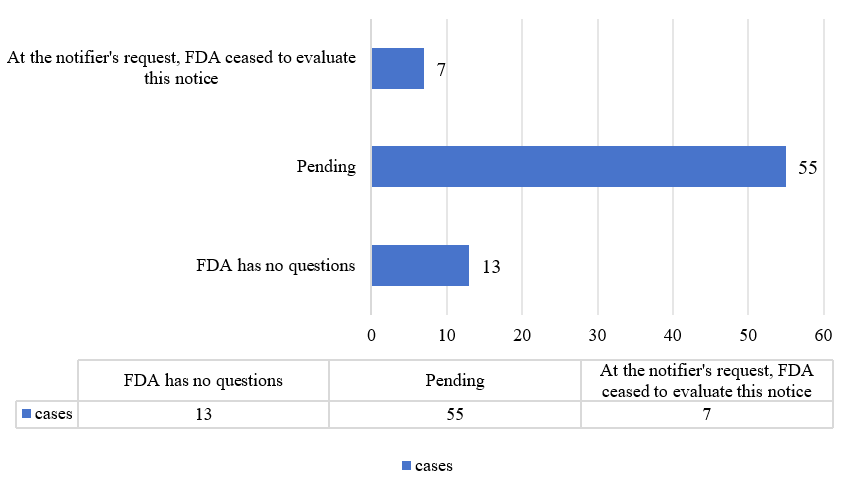
Figure 1. Overview of GRAS notifications in 2024
2. Substances notified in 2024 (based on approval dates): 13 substances
In 2024, 13 substances were notified, including five microorganism-related substances, three sugar substitute ingredients, two dairy products, two proteins, and one 1-methylcyclopropene compound.
Compared to 2023, there has been a declining trend in the number of substances obtaining GRAS notifications (details can be seen in the article Surge in Applications: Overview of FDA GRAS Notifications from 2021 to 2023).
CIRS speculates that, on the one hand, the increasing number of FDA GRAS submissions in recent years has impacted the review speed. On the other hand, the FDA has been imposing stricter review requirements for submitted substances. For example, many new substances produced using synthetic biology technologies now require more detailed and comprehensive documentation.
Table 1. Substances obtained the FDA GRAS notice in 2024
No. | GRN No. | Substance | Notified date | Enterprise |
1 | 1057 | D-psicose | 2024/1/25 | Tate & Lyle (Britain) |
2 | 1125 | Pea protein fermented by shiitake mycelia | 2024/3/27 | MycoTechnology, inc. (US) |
3 | 1127 | Lactiplantibacillus plantarum ATCC-202195 | 2024/2/1 | Danisco USA, Inc. (US) |
4 | 1132 | Hydrolyzed poultry protein | 2024/1/29 | Norilia AS (Norway) |
5 | 1134 | Bacteriophage preparation specific to Salmonella Enteritidis | 2024/2/8 | Qingdao Phagepharm Biotech Co., Ltd. (China) |
6 | 1137 | Protein-sucrose | 2024/1/19 | Incredo Ltd. (Israel) |
7 | 1142 | Brazzein | 2024/3/11 | Oobli, Inc. (US) |
8 | 1143 | Bacillus subtilis NRRL 68053 | 2024/1/19 | Microbial Discovery Group (US) |
9 | 1146 | Nuclease enzyme preparation produced by Bacillus amyloliquefaciens expressing the gene encoding a nuclease from Serratia marcescens | 2024/6/12 | c-LEcta GmbH (Germany) |
10 | 1155 | 1-methylcyclopropene | 2024/4/23 | Fresh Inset S.A. (Poland) |
11 | 1158 | Lactiplantibacillus plantarum DSM 34613 | 2024/5/8 | Scott Laboratories, Inc. (US) |
12 | 1172 | Liquid milk, either whole or nonfat, combined with lactose and water | 2024/5/20 | Synlait Milk Limited (New Zealand) |
13 | 1179 | Liquid whole cow milk | 2024/9/24 | Crossway Foods, Ltd. (Ireland) |
3. Substances still pending in 2024
In 2024, 57 substances were submitted for FDA GRAS notification, of which two have been notified while 55 are still pending. Notably, due to the surge in submissions, the FDA has extended the time required to archive newly submitted dossiers (assigning GRN numbers). As a result, many products submitted in 2024 have not yet been archived or, if archived, have not been updated on the official website.
14 substances submitted in 2024 by Chinese companies are currently pending. Among them, seven are alternative sweeteners (four steviol glycosides, two D-psicose, and one brazzein preparation), one hydroxytyrosol, one algal oil, one fungal oil, one inositol, one ergothioneine, one microorganism-related product, and one human milk oligosaccharides (HMOs).
Table 2. Substances submitted by Chinese companies still pending (based on publicly available information)
No. | GRN No. | Substance | Enterprise |
1 | 1178 | Rebaudioside I obtained by enzymatic treatment of steviol glycosides purified from the leaves of Stevia rebaudiana (Bertoni) Bertoni (rebaudioside I) | Sichuan Ingia Biosynthetic Co., Ltd |
2 | 1182 | Hydroxytyrosol | Hangzhou Viablife Biotech Co, Ltd. |
3 | 1184 | Rebaudioside M from a modified strain of Escherichia coli BL21 (DE3) | Sichuan Ingia Biosynthetic Co., Ltd. |
4 | 1185 | Algal oil (≥35% docosahexaenoic acid) from Schizochytrium sp. FJRK-SCH3 | Runke Bioengineering (Fujian) Co., Ltd. |
5 | 1186 | Fungal oil (≥38% arachidonic acid (ARA)) from Mortierella alpina FJRK-MA01 | Runke Bioengineering (Fujian) Co., Ltd. |
6 | 1188 | D-psicose | Shandong Starlight So True Biological Technology Co., Ltd |
7 | 1191 | Ergothioneine produced by Escherichia coli BL-21 (DE3) expressing ergothioneine synthases from Schizosaccharomyces pombe | Shanghai EGT Synbio Group Co., LTD |
8 | 1193 | D-psicose | Sichuan Ingia Biosynthetic Co., Ltd. |
9 | 1198 | Inositol | Sichuan Bohaoda Biological Technology Co., Ltd. |
10 | 1203 | Rebaudioside M2 obtained by enzymatic treatment of steviol glycosides purified from the leaves of Stevia rebaudiana (Bertoni) Bertoni | Guilin Layn Natural Ingredients Corp. |
11 | 1204 | Lacticaseibacillus casei Zhang | Beijing Scitop Bio-tech Co., LTD |
12 | 1206 | Rebaudioside M produced by enzymatic treatment of rebaudioside A purified from the leaves of Stevia rebaudiana (Bertoni) Bertoni | Adorvia Biotechnology Co., Ltd. |
13 | 1207 | Brazzein preparation produced by Aspergillus oryzae 90402 expressing a gene encoding for brazzein from Pentadiplandra brazzeana | Nanjing Bestzyme Bio-Engineering Co. Ltd. |
14 | 1208 | Lacto-N-triose II | Shandong Henglu Biotechnology Co., Ltd. |
4. Popular GRAS substances in 2024
In 2024, a wide variety of products have been submitted for the FDA GRAS notification, with microbial-related products being particularly popular. Sugar substitutes such as steviol glycosides, brazzein, D-psicose, and monocin preparations, as well as nutritional substances like HMOs and dairy products, continue to be in high demand for submission.
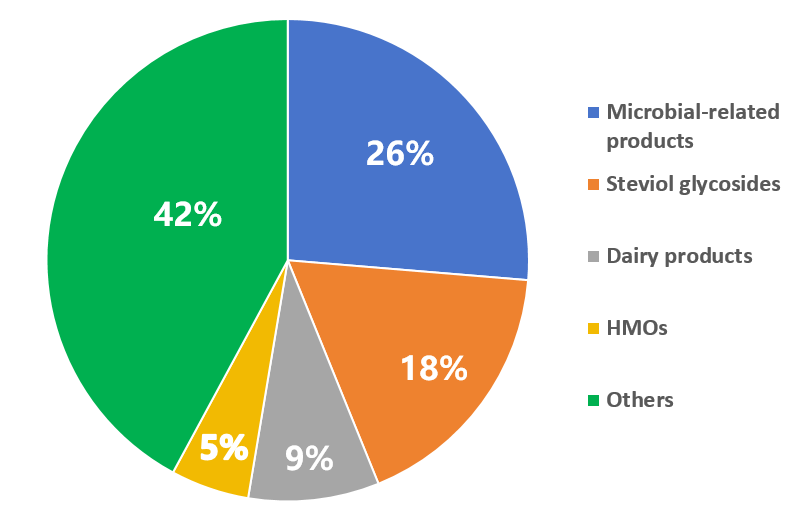
Figure 2 Proportion of product categories submitted for the FDA GRAS notice in 2024
Table 3. Details of popular substances in 2024
Category | Substances | Number of notified | Number of pending notice | Total |
microbial-related products | Enzyme preparations | 1 | 8 | 9 |
Lactiplantibacillus plantarum | 2 | 2 | 4 | |
Bacteriophage preparations | 1 | 2 | 3 | |
Bacillus subtilis | 1 | 1 | 2 | |
Saccharomyces cerevisiae | 0 | 1 | 1 | |
Lacticaseibacillus casei | 0 | 1 | 1 | |
Total | 5 | 15 | 20 | |
steviol glycosides | D-psicose | 1 | 2 | 3 |
Brazzein preparation | 1 | 2 | 3 | |
Rebaudioside M | 0 | 2 | 2 | |
Protein-sucrose | 1 | 0 | 1 | |
Rebaudioside M2 | 0 | 1 | 1 | |
Rebaudioside I | 0 | 1 | 1 | |
Monocin preparation | 0 | 1 | 1 | |
Cellobiose | 0 | 1 | 1 | |
Total | 3 | 10 | 13 | |
dairy products | Whole dry sheep milk | 0 | 2 | 2 |
Liquid milk, including whole and skim milk, mixed with lactose and water | 1 | 0 | 1 | |
Liquid whole cow milk | 1 | 0 | 1 | |
Liquid whole goat milk | 0 | 1 | 1 | |
Total | 2 | 3 | 5 | |
HMOs | 6'-SL | 0 | 1 | 1 |
LNT | 0 | 1 | 1 | |
LNnT | 0 | 1 | 1 | |
Total | 0 | 3 | 3 | |
Others | Spore preparation | 0 | 3 | 3 |
Algal oil | 0 | 3 | 3 | |
Pea protein | 1 | 0 | 1 | |
Hydrolyzed poultry protein | 1 | 0 | 1 | |
1-Methylcyclopropene complex | 1 | 0 | 1 | |
Saxifrage powder | 0 | 1 | 1 | |
Resistant dextrin | 0 | 1 | 1 | |
Polypeptides | 0 | 1 | 1 | |
Ovotransferrin | 0 | 1 | 1 | |
Inositol | 0 | 1 | 1 | |
Rhamnogalacturonan | 0 | 1 | 1 | |
β-Lactoglobulin | 0 | 1 | 1 | |
Goat lactose | 0 | 1 | 1 | |
Goat whey | 0 | 1 | 1 | |
Fungal oil | 0 | 1 | 1 | |
Ergothioneine | 0 | 1 | 1 | |
Recombinant bovine lactoferrin isolate | 0 | 1 | 1 | |
Protein preparation | 0 | 1 | 1 | |
Hydroxytyrosol | 0 | 1 | 1 | |
Galacto-oligosaccharides | 0 | 1 | 1 | |
Chemically synthesized pediocin PA-1 analog | 0 | 1 | 1 | |
(R)-1,3-Butanediol | 0 | 1 | 1 | |
Protein Hydrolysate | 0 | 1 | 1 | |
Total | 3 | 24 | 27 |
5. Summary
In 2024, the FDA GRAS notice reflects the food industry’s pursuit of both innovation and safety. Although the number of notified substances has declined, the submission activity remains high. Chinese companies are significantly increasing their participation in the fields of functional ingredients and biosynthetic substances.
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.
*Data Source: The inventory of GRAS notices and the latest released accepted GRAS dossiers (updated as of 2025/01/07).
*Note: Because the FDA doesn’t disclose dossier acceptance dates, the statistics for substances currently pending are primarily based on the submission dates recorded in the released dossiers.
The statistics are based on the published dossiers with GRN No., and are for reference only.

