According to data from the SAMR's Special Food Query Platform, five imported health food products secured registration approvals in the summer of 2025. This marks the first such approvals granted in seven years.
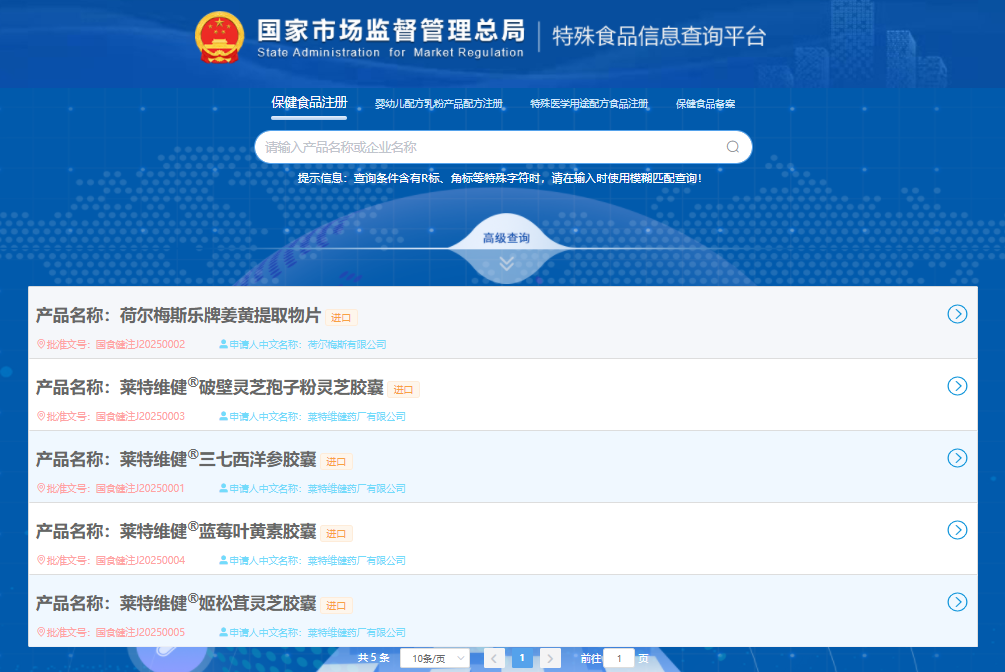
CIRS Group has collected the basic information about the five approved products, as shown in Table 1 below. The five approved products came from two companies, respectively, with four from Hong Kong, and one from Indonesia.
Table 1. Details of the five registered imported health food products
Chinese Name | Register No. | Applicant | Health Function |
萊特維健®Notoginseng & American Ginseng Capsules | 國食健注 J20250001 | PHARMTECH (HONG KONG) LIMITED | Strengthen immunity |
荷爾梅斯樂牌Curcumin Tablets | 國食健注 J20250002 | PT. HELMIGS PRIMA SEJAHTERA | Maintain a healthy level of blood lipid and has an auxiliary protective effect on chemical liver injury |
萊特維健®Ganoderma Lucidum Spore Powder (Broken Shell) Capsules | 國食健注 J20250003 | PHARMTECH (HONG KONG) LIMITED | Strengthen immunity |
萊特維健®Blueberry & Lutein Capsules | 國食健注 J20250004 | PHARMTECH (HONG KONG) LIMITED | Relieve visual fatigue |
萊特維健®Agaricus blazei & Ganoderma lucidum Capsules | 國食健注 J20250005 | PHARMTECH (HONG KONG) LIMITED | Strengthen immunity |
According to the Guide to Imported Health Food (2025 Edition) compiled by CIRS Group, since 2018, the registration of imported health foods has been stuck in a regulatory freeze with zero approvals. This situation is attributed not only to the diversion of the filing system (which reduced the need for full registrations), but also to the slow implementation of regulations and the practical hurdles from the on-site inspection requirements.
The recent approval of five imported health food products sends a clear positive signal: As long as the registration materials are complete and compliant during the acceptance and review processes, China remains fully open to importing these products!
About CIRS Group
Established in 2012, the Food Business Division of CIRS Group has helped over 1,000 domestic and international food companies achieve one-stop compliance solutions. CIRS offers a full range of regulatory services covering novel food applications, synthetic biology-derived foods, U.S. GRAS notice, EU novel food application, health food registration, and food for special medical purposes (FSMP).
Our food services in China include but not limited to:
- China new food raw materials registration
- China new food additive registration
- China health food (dietary supplement) registration/filing
- China health food testing service
- China new food contact substance registration
- China food for special medical purpose (FSMP) registration
- China infant formula milk powder registration
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.
This article was updated on August 21, 2025, to correct some minor errors.

