In China, health food is usually defined as food product that have specific health function or supply vitamins and (or) minerals. With the goal of regulating body's function, health food is suitable for specific groups of people. However, it is not used for the purpose of curing disease and causes no acute, sub-acute or chronic health effect to human body.
The Definitions/Names of these Food Products in Different Countries are Different:
China: Health Food
EU: Food Supplement
USA: Dietary Supplement
Canada: Natural Health Product
Australia: Complementary Medicines
Korea: Health Functional Food
Japan: Food with Health Claims (FHC)
Classification of Health Food (Dietary Supplement)
I. Nutrition supplement:
Food that replenishes the vitamins and (or) minerals but without providing energy or other active ingredients.
II. Functional health food:
Food that labeled with health function claim has physiological effects on the human body.
Regulation Background
In accordance with Food Safety Law of the People’s Republic of China (2015 version), companies who plan to place health food in Chinese market shall apply and obtain the health food registration certificate or filing certificate. For domestic health foods produced in China, the registration shall be conducted with State Administration for Market Regulation (SAMR, former CFDA), whereas, the filing shall be carried out with Provincial Administration for Market Regulation. For imported health foods produced in oversea factories, both the registration and filing shall be applied with SAMR. Meanwhile, oversea companies shall have a permanent Chinese representative office or appoint a Chinese agent to deal with registration or filing and obtain such certificates.
Relevant Regulations on Health Food (Dietary Supplement)
Name | Released Date | Implemented Date |
|---|---|---|
Food Safety Law of the People's Republic of China (2015 version) | 2015.04.24 | 2015.10.01 |
Administrative Measure on Health Food (Dietary Supplement) Registration and Filing | 2016.03.01 | 2016.07.01 |
National Standard for Health Food (Dietary Supplement) (GB 16740-2014) | 2014.12.24 | 2015.05.24 |
2015.07.28 | - | |
2016.11.17 | 2016.11.17 | |
Health Food (Dietary Supplement) Registration Application Service Guideline | 2016.12.23 | 2016.12.23 |
Health Food (Dietary Supplement) Raw Materials Directory (the first batch) | 2017.01.12 | 2017.01.12 |
Directory of Health Function Available to Claim for Health Food (the first batch) | 2017.01.12 | 2017.01.12 |
2017.05.02 | 2017.05.02 | |
Available Excipients for Health Food (Dietary Supplement) Filing and Their Usage Rules (Trial) | 2017.05.02 | 2017.05.02 |
Main Production Processes of Health Food (Dietary Supplement) Filing Products (Trial) | 2017.05.02 | 2017.05.02 |
Health food registration and filling shall be carried out according to Administrative Measure on Health Food Registration and Filing (herein named the Measure). The Detailed information are as follows:
Part I Health Food (Dietary Supplement) Filing
(People like to call it “Red/Orange Hat”, in fact, once the filing certificate is received, the logo of “Blue Hat” rather than “Red/Orange Hat” will be put on the label)
Applicable Scope of Filing
Nutrition Supplement: The health food whose formulation meet the requirements in Health Food Raw Materials Directory and Available Excipients for Health Food Filing and Their Usage Rules (Trial) can do the filing rather than registration.
Applicant's Qualification of Filing
I. The filing applicant of domestic health food shall be the factory who has the production certificate. In other word, the domestic applicant cannot entrust production.
II. The filing applicant of imported health food could be the oversea manufacturer (oversea manufacturer refers to the legal person and other organization). In other word, the oversea applicant could entrust production.
Filing Procedure
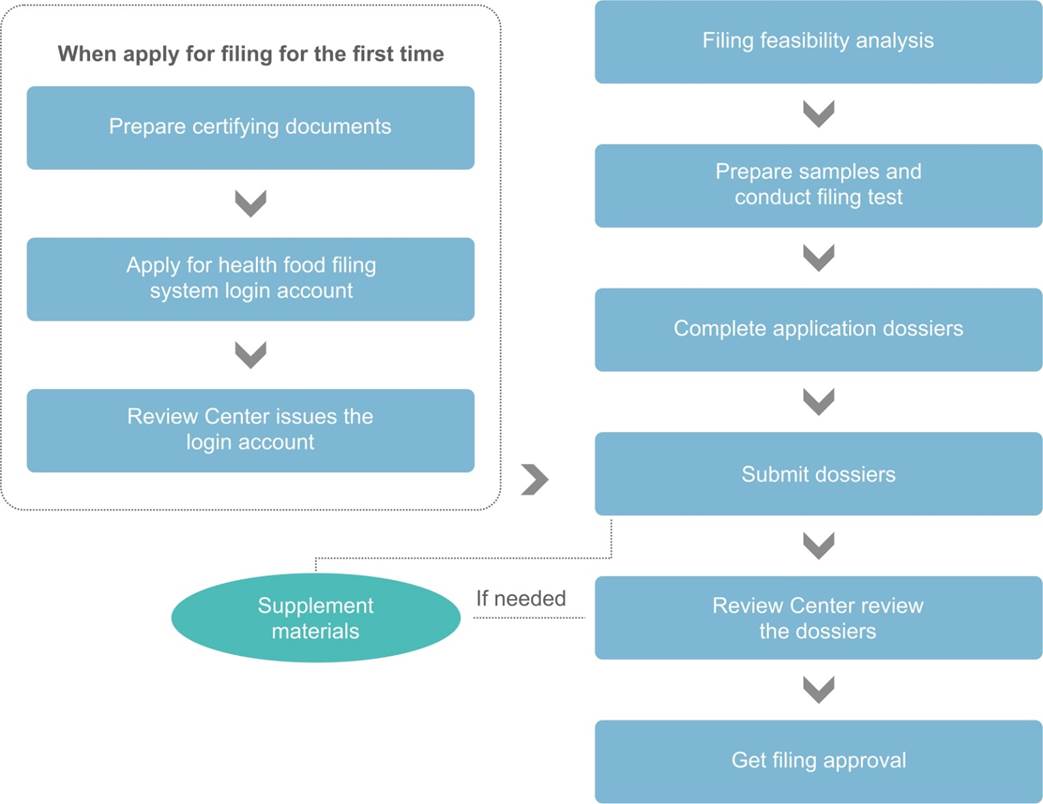
Dossier Requirements for Health Food (Dietary Supplement) Filing System Login Account Application
If it is the first time to apply for filing, the applicant shall get the health food filing system login account in advance. The required documents are as following:
Basic company information and contact information;
Qualification certifying document of the applicant;
Letter of Authorization of the contact;
Scanned passport/ identification card of legal representative.
Dossier Requirements of Filing
According to the Measure, the following documents are required for health food filing:
Health food filing application form; Letter of commitment for authenticity of the materials;
Copies of legally registered certificates of the applicant;
Product formulation materials (APIs and excipients);
Product production process materials;
Stability test report, and the explanation of the use of active ingredient and excipients;
Information of packaging materials in direct contact with the product;
Samples of product label and package insert;
Product technical requirements;
Test according to product technical requirements;
Other materials proving product safety and health function.
For imported health food filing, besides the above documents, the following supplementary documents also should be submitted:
Qualification certifying documents issued by government authorities or legal service agencies in the producing country (region) of origin proving that the oversea filing applicant is the owner of the health food marketed;
Certifying documents issued by government authorities or legal service agencies in the producing country (region) of origin proving that the product has been marketed more than a year, or safety report of oversea sales and consumer’s feedback;
Health food-associated standards issued by the product producing country (region) of origin or international organizations;
Packaging, labels, package inserts for products marketed in the producing country (region)of origin;
For filing affairs run by oversea manufacturer’s Permanent Representative in China, a copy of the "registration certificate of oversea enterprise’s permanent Chinese representative offices" shall be provided; for filing affairs run by domestic agencies entrusted by oversea manufacturers, the applicant shall provide the original notarized certificate of entrustment and copies of business license of the agencies entrusted.
Test Requirements of Filing
According to the Measure, the following tests are required to be arranged in CFDA designated testing institutions for health food filing:
Tests listed in the document of technical requirements including Functional components/characteristic ingredients test, Hygiene health test, etc. for three batches of samples;
Stability test for three batches of samples.
Part II Health Food (Dietary Supplement) Registration
(Once the registration certificate is received, the logo of “Blue Hat” can be put on the label)
Applicable Scope of Registration
Functional Health Food: The health food whose active ingredients are out of the scope of Health Food Raw Material Directory. And it has some specific health function for specific groups of people.
27 Approved Health Function
1 | 2 | 3 | 4 |
|---|---|---|---|
Enhancing immune | Assisting blood lipids reduction | Assisting blood sugar reduction | Anti-oxidative |
5 | 6 | 7 | 8 |
Assisting memory improvement | Alleviating eye fatigue | Alleviating lead excretion | Clear the throat |
9 | 10 | 11 | 12 |
Assisting blood pressure reduction | Sleep Improvement | Facilitating milk secretion | Alleviating physical fatigue |
13 | 14 | 15 | 16 |
Enhancing anoxia endurance | Assisting irradiation hazard protection | Weight loss | Improving child growth |
17 | 18 | 19 | 20 |
Increasing bone density | Improving nutritional anemia | Assisting the protection against chemical injury of liver | Eliminating acne |
21 | 22 | 23 | 24 |
Eliminating skin chloasma | Improving skin water content | Improving skin oil content | Regulating gastrointestinal tract flora |
25 | 26 | 27 | |
Facilitating digestion | Facilitating feces excretion | Assisting the protection of gastric mucosa |
Note: The three health functions highlighted in RED maybe canceled in future.
Applicant's Qualification of Registration
I. The registration applicant of domestic health food could be the legal person or other organization registered in China. In other word, the domestic registration applicant could entrust production.
II. The registration applicant of Imported health food could be the oversea manufacturer (oversea manufacturer refers to the legal person and other organization). In other word, the oversea registration applicant could entrust production.
Registration Procedure
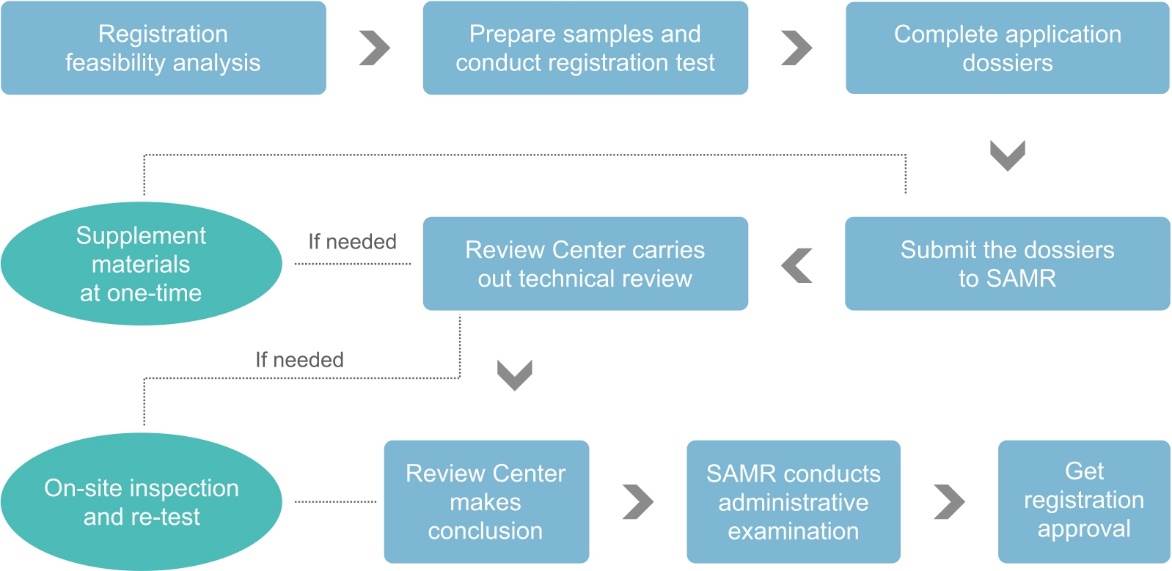
Dossier Requirements of Registration
According to the Measure, the following documents are required for health food registration:
Health food registration application form; Letter of commitment for authenticity of the materials;
Copies of legally registered certificates of the applicant;
Product development report including the product technical requirements;
Product formulation materials (APIs and excipients);
Product production process materials;
Safety and function assessment material; and tests reports of functional components/characteristic ingredients, stability, hygiene health and others if necessary;
Information of packaging materials in direct contact with the product;
Samples of product label and package insert;
3 samples with the minimum sales packaging;
Other materials pertaining to the product registration technical evaluation.
For imported health food registration, besides the above documents, the following supplementary documents also should be submitted:
Qualification certifying documents issued by government authorities or legal service agencies in the producing country (region) of origin proving that the oversea registration applicant is the owner of the health food marketed;
Certifying documents issued by government authorities or legal service agencies in the producing country (region) of origin proving that the product has been marketed more than a year, or safety report of oversea sales and consumer’s feedback;
Health food-associated standards issued by the product producing country (region) of origin or international organizations;
Packaging, labels, package inserts for products marketed in the producing country (region)of origin;
For registration affairs run by oversea manufacturer’s Permanent Representative in China, a copy of the "registration certificate of oversea enterprise’s permanent Chinese representative offices" shall be provided; for registration affairs run by domestic agencies entrusted by oversea manufacturers, the applicant shall provide the original notarized certificate of entrustment and copies of business license of the agencies entrusted.
Test Requirements of Registration
According to the Measure, the following tests are required to be arranged in CFDA designated testing institutions for health food registration:
Safety and toxicology test;
Animal and (or) human function test;
Functional components/characteristic ingredients test, Hygiene health test;
Stability test;
Strain identification and strain virulence test for Probiotics-based Health Food;
Stimulants, illicit drugs test for Health Food with Relieving physical fatigue, Weight loss or Improving growth and development function;
Other tests if necessary
Our Services
CIRS is providing one-stop services of China food regulatory compliance. We also deliver the most up-to-date regulatory information about food safety control in China. For health food, we offer the following services:
I. Training on Health Food Regulation
II. Health Food Regulation Update Monitoring
III. Health Food Registration
IV. Health Food Filing
V. Single Technology Services
Pre-market Investigation
Classification Analysis and Formula Review
Chinese Label and Package Insert Design
Dossier Preparation
Translation
Test Arrange and Monitoring

