On May 2, 2025, the Scientific Committee on Consumer Safety (SCCS) of the European Union issued scientific advice (SCCS/1675/25) on Methyl Salicylate (CAS No. 119-36-8), which is the revision of the scientific advice issued on September 22, 2023 (SCCS/1654/23).
Scientific Advice on Methyl Salicylate - Children’s Exposure
1. Taking into consideration the conclusions of SCCS/1658/23 and the aggregate exposure, the SCCS is requested to reassess the maximum concentration of Methyl Salicylate that is considered safe when used in products intended for children aged 0-3.
The SCCS is of the view that, to be considered safe, the concentration of Methyl Salicylate should not exceed 0.4% in toothpaste and 0.02% in other products when used in products intended for children aged 0-3 years.
2. The SCCS mandates do not address environmental aspects. Therefore, this assessment did not cover the safety of Methyl Salicylate for the environment.
Regulatory Requirements Interpretation of Methyl Salicylate
The Global Cosmetic Ingredient Regulatory Database – Global CosIng, an upgraded version of China CosIng independently developed by CIRS Group, indicates that Methyl Salicylate has been included in the following key lists globally.
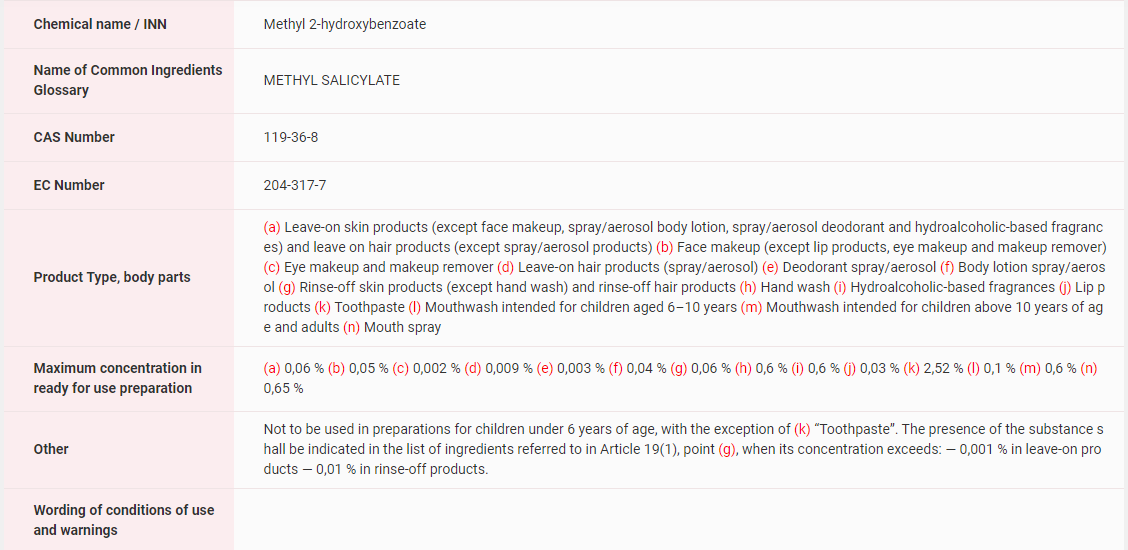
In EU, Methyl Salicylate has been included in the List of substances which cosmetic products must not contain except subject to the restrictions laid down, detailed information about product type, body parts and maximum concentration in ready for use preparation can be found in the figure below.
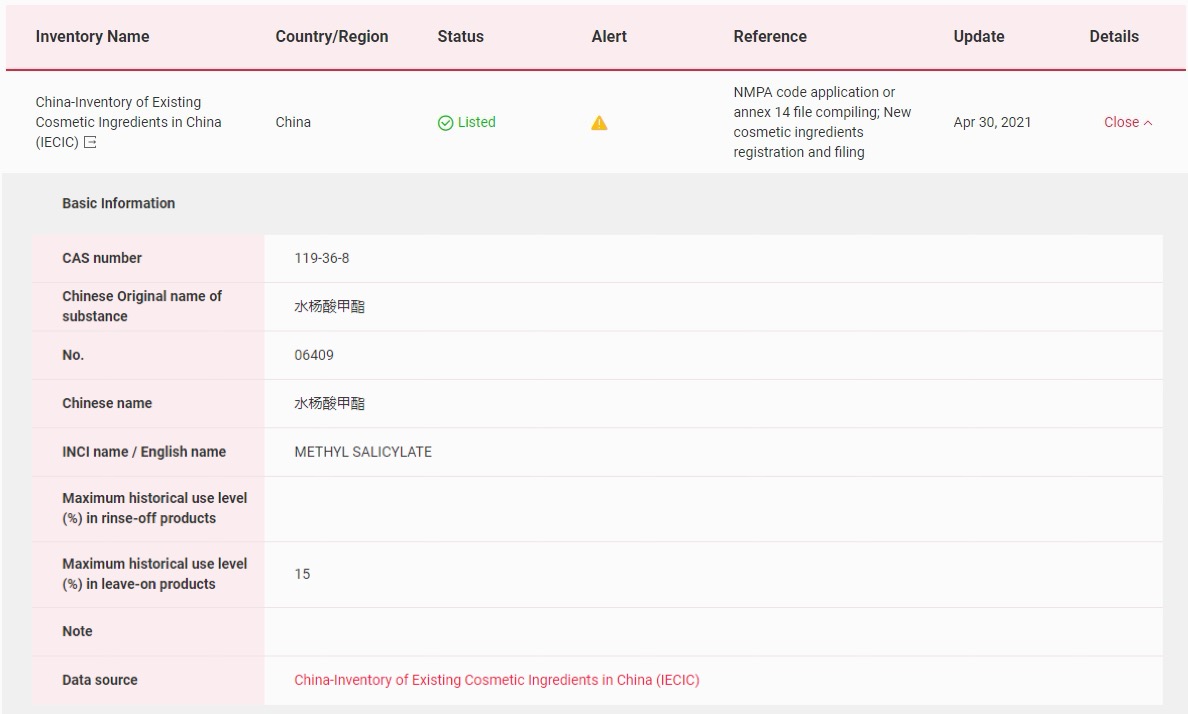
In China, Methyl Salicylate has been included in the Inventory of Existing Cosmetic Ingredients in China (IECIC), the maximum historical use level (%) in leave-on products is 15%.
About CIRS
The CIRS cosmetic team is dedicated to ensuring that cosmetic products meet stringent global regulatory standards. It can provide one-stop services covering the whole life-cycle of a personal care product, which includes cosmetic ingredient development, physical/chemical tests, toxicological tests (in vivo & in vitro), efficacy studies (in vivo & in vitro), ingredient registration, and product registration.
Our Services
- EU Cosmetic Product Formula and Label Review;
- EU Cosmetic Responsible Person;
- Product Information File (PIF); and
- EU Cosmetic Safety Assessment (CPSR)
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.
Further Information

