On 17 Jan, 2018, CFDA published the “Guidelines for Cosmetic Classifications (Drafted Version)” and request for public feedbacks of any suggestions on amendments to be sent via email to CFDA’s Department of Cosmetic Registration before 30 Jan, 2018.
Contact No.: 010-88330729
Email: hzpc@sfda.gov.cn
Background
On 15 Mar, 2016, the National Institutes for Food and Drug Control published the
“Standards for Cosmetic Classifications (Drafted Version)” and seek public
opinions on this matter. According to the current cosmetic regulation, cosmetics
are mainly divided into non-special use and special use products according to
their functions. Based on the principle of risk management, and under the
premise of ensuring products’ quality and safety, CFDA’s Department of Cosmetic
Registration carried out the study of classification of cosmetics. The
classifications are mainly refined and optimized according to the following
aspects:
1) Establish the classification principle of cosmetics based on efficacy claiming, application site, form, suitable users and safety risks;
2) Use coding system; and
3) Adopt the mode of open classification table managemen;
Main contents of Guidelines for Cosmetic Classifications (Drafted Version)
Standards for
cosmetic classifications: product’s efficacy claim, application site, product
form and suitable users
Coding structure:
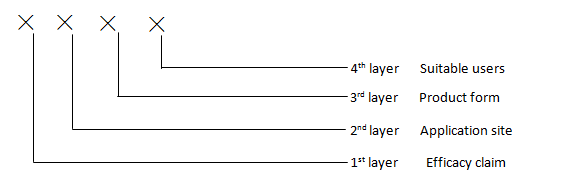
Coding methods:
- The codes from 1 to 4 layers are sequenced together with “-“s in between
- Each layer is represented by 2 or more digit numbers, with coding from 00– 99. For multi efficacy clamming, application sites or varies suitable users, coding can be sequenced in accordance with their degree of risk
Code for 1st layer (Efficacy claim): 00 - 25
Code for 2nd layer (Application site): 00 – 25 (Note: N/A for oral cavity (dental and oral mucosa))
Code for 3rd layer (form): 00 - 17
Code for 4th layer (suitable users): 00 - 06
Comparison between “Guidelines for Cosmetic Classifications (Drafted Version)” and “Standards for Cosmetic Classifications (Drafted Version)”
Coding Structure
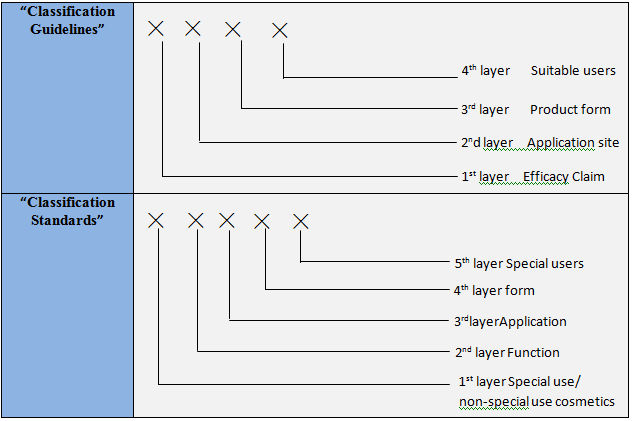
Coding method
“Classification Guidelines”: represented by numbers
“Classification Standards”: represented by
numbers and letters
Efficacy claiming
“Classification Guidelines” | “Classification Standards” |
Hair Growth | Hair Growth |
Hair Dye | Hair Dye |
Hair Perm | Hair Perm |
Depilation | Depilation |
Breast Shaping | Breast Shaping |
Body Shaping | Body Shaping |
Deodorant | Deodorant |
Whitening and Anti-freckle | Whitening and Anti-freckle |
Sunscreen | Sunscreen |
Anti-wrinkle | Anti-wrinkle |
Anti-acne | Anti-acne |
Oil control | Oil control |
Anti-dandruff | Anti-dandruff |
Exfoliator | Exfoliator |
Talcum Powder | Talcum Powder /bath powder |
Cleansing | Cleansing |
Make-up Removal | Make-up Removal |
Moisturizing | Moisturizing |
Nourishing | Nourishing |
Repairing | Repairing |
Beautify | Beautify |
Perfume | Perfume |
Hair Styling | Hair Styling |
Foundation | |
Pore Cleansing | |
Others | Anti-aging |
Application site
“Classification Guidelines” | “Classification Standards” |
Hair | Hair |
Scalp | Scalp |
Skin | Body skin (including facial skin) |
Body | Body Skin (excluding facial skin) |
Facial (excluding mucosa) | Facial (excluding mucosa) |
Eye area | Eye area |
Lip | Lip |
Nose | |
T-region | |
Back of ear | |
Hair line region | |
Shaving area | Shaving area |
Eyebrow | Eyebrow |
Eyelash | Eyelash |
Facial hair | |
Neck | |
Shoulder | |
Breast | |
Armpit | |
Hand | Hand |
Foot | Foot |
Finger (toe) nail | Finger (toe) nail |
Body Hair (including armpit) | Body Hair (including armpit) |
Body Hair (excluding armpit) | Body Hair (excluding armpit) |
Hip Others | |
Oral cavity (dental and oral mucosa) | Oral cavity (dental and oral mucosa) |
Form
“Classification Guidelines” | “Classification Standards” |
Cream | Cream |
Emulsion | Emulsion |
Aqua | Aqua |
Gel | Gel |
Oil | Oil |
Powder | Powder |
Mud | Mud |
Spray | Spray |
Aerosol | Aerosol |
Filming | Filming |
Wax base | Wax base |
Organic solvent | Organic solvent |
Capsule | Capsule |
Multi-phase | |
Blending agent | |
Tablet | |
Wipes | |
Others |
Suitable users
“Classification Guidelines” | “Classification Standards” |
General user | |
Pregnant women | Pregnant women or breast feeding women |
breast feeding women | |
Children | Children |
Men | |
Elderly | Elderly |
Others |
CIRS comments:
1). The classification of cosmetics will be refined from 5 categories of non-special use products and 9 categories of special use products to 26 categories of efficacy claiming, 27 categories of application site, 18 categories of product forms and 7 categories of suitable users with coding.
2). Compared to the first edition of the draft version of “Standards for Cosmetic Classifications”, the second draft version of “Guidelines for Cosmetic Classifications” further optimized and refined the classification of cosmetics in efficacy claiming, application site, product form and suitable users.
3). Compared to the current classification standard of cosmetics in the US, EU, Japan and Korea, this guideline for classification is the most detailed and complex. Due to China’s current cosmetic regulation’s requirement of safety data, supporting documents of efficacy claiming, suitable advertising wording that can be used in different efficacy claiming, exposure site and safety assessment of exposure group might also be taken into consideration. This classification guideline is beneficial to the competent authorities to conduct more targeted and accurate pre-, in- and post-market supervision. However, in combination with the current situation of China's cosmetic industry, the competent authorities, cosmetic manufacturers and cosmetic raw material suppliers will all face huge challenges.
If you have any needs or questions, please contact us at service@cirs-group.com.
Source: New Guidelines for Cosmetic Classification (draft for public comments)

