On 18 May 2016, China Health Food Registration and Evaluation Centre announced that 331 products registration applications, of which the supplementary materials hadn’t been submitted by due date, would be returned in case enterprises failed to hand in written objections or appropriate reasons. This notification is an alarm for relevant enterprises.
Analysis result of returned health foods
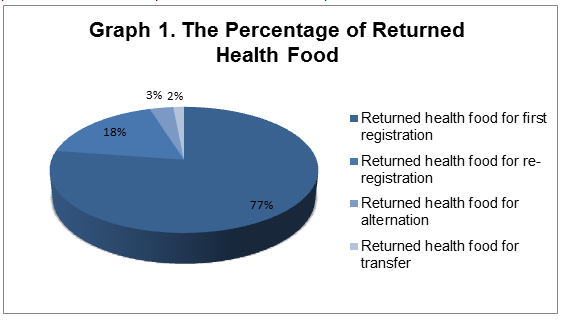
1. 331 returned health food products includes 256 first registration products, 59 re-registration products and 11 alteration products as well as 5 transfer products.
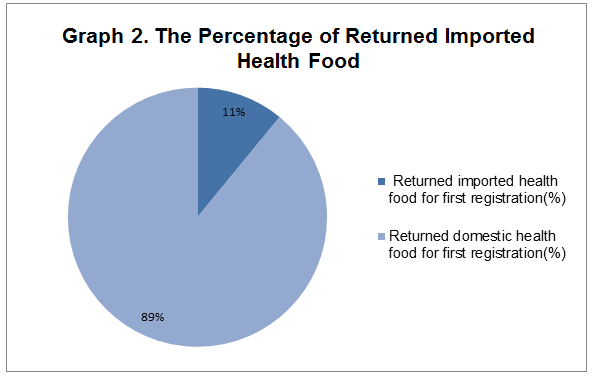
2. In the 256 returned health food for first registration, there are 29 imported health food products, which share 11 percent of total returned first registration products.
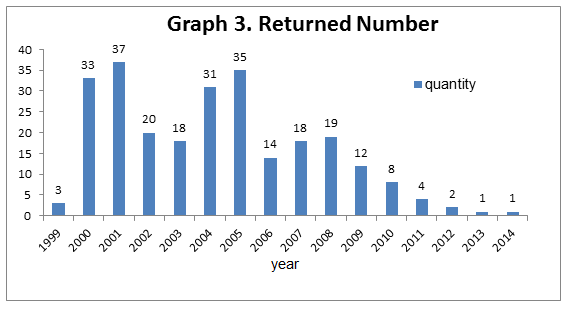
3. Most of the returned first registration health food products are applied before 2005, especially 2005, 2001 and 2000, and they have been decreased significantly in recent five years (2011~2016).
4. Note:
4.1 Returned supplement food products cannot submit supplementary materials directly. They shall reapply the registration based on health food registration procedure. When enterprises hand in registration application again, they shall provide reasons for re-registration with Not Approval Notification and the reasons shall contain modified items, modified details and modified reasons.
4.2 If the modification of re-registration product influences product function, food safety or product quality, it is required to conduct testing again.
Time limit for material supplementing
Analysis result of returned health foods
1. 331 returned health food products includes 256 first registration products, 59 re-registration products and 11 alteration products as well as 5 transfer products.
2. In the 256 returned health food for first registration, there are 29 imported health food products, which share 11 percent of total returned first registration products.
3. Most of the returned first registration health food products are applied before 2005, especially 2005, 2001 and 2000, and they have been decreased significantly in recent five years (2011~2016).
4. Note:
4.1 Returned supplement food products cannot submit supplementary materials directly. They shall reapply the registration based on health food registration procedure. When enterprises hand in registration application again, they shall provide reasons for re-registration with Not Approval Notification and the reasons shall contain modified items, modified details and modified reasons.
4.2 If the modification of re-registration product influences product function, food safety or product quality, it is required to conduct testing again.
Time limit for material supplementing
| Administrative Measures on Health Food Registration (Trial) | Administrative Measures on Health Food Registration and Record, which will come into force on July 1, 2017 |
| Enterprises should submit their supplementary materials at one time within five months as required by the Notice of Deficiency. In case of special reasons, the time limit may be postponed (seven months longer at most). | Enterprises should submit the supplementary materials at one time within three months as required by the Notice of Deficiency. In case of objection, enterprises should apply for re-examination in written forms within 20 working days upon the receipt of the Notice. Reasons for the re-examination should also be given. |
CIRS Comments
- As long-time review and evaluation for the registration of health food is disadvantageous for the development of food industry, CFDA has always been trying to find a solution to increase technical evaluation efficiency. As a result, CFDA released this notice on returned food for purpose to reduce review and evaluation pressure by eliminating products with disqualified declaration materials.
- Health food enterprises should be vigilant and avoid following suit to declare their products blindly; enterprises should also improve the professional quality of declare persons as well as trying to make their declaration materials more professional and their products better compliance with relevant laws and regulations.
- In the future, the time limit for submitting supplementary materials for the registration of health food will become shorter; requirements for the materials also become stricter. Adulterated information will never be tolerated. In order to ensure the scientific base of products, enterprises should enhance their researching and developing capability.
Source:http://www.zybh.gov.cn/d?xh=219856
Contact us
Ms. Cathy Yu Team Leader of Food Safety and Regulatory Affairs Department, CIRS China
11F Dongguan Building, 288 Qiuyi Road, Binjiang District, Hangzhou, China, 310020
Tel: +86 571 8720 6538 | Fax: +86 571 8720 6533
Email: cathy.yu@cirs-group.com






